Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA)
- PMID: 18925902
- PMCID: PMC2899484
- DOI: 10.1111/j.1600-6143.2008.02417.x
Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA)
Abstract
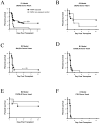
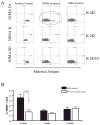
Developmental exposure to noninherited maternal antigens (NIMA) exerts a tolerizing or sensitizing influence on clinical transplantation in humans and experimental animals. The aim of this study was to determine if strain and gender differences influence the NIMA effect. Six different mouse strain backcross matings of F(1) females with homozygous males ('NIMA backcross') and corresponding control breedings of F1 males with homozygous females were performed. H-2 homozygous offspring underwent heterotopic heart transplantation from fully allogeneic donors expressing noninherited H-2 antigens. A NIMA tolerizing effect on heart allograft outcome was found in three of six breeding models. In all three cases, the tolerizing antigens were from an H-2(d+) strain. The tolerogenic effect was greatest in male as compared with female recipients. Offspring from the three breeding models in which no tolerance was seen, appeared to be sensitized based on poorer graft survival, or enhanced T- or B-cell responses to the noninherited H-2(b or k) antigens. Significantly higher percentages of maternal antigen(+) cells were found in the peripheral blood of tolerant versus nontolerant strains of backcross mice prior to transplant. Our findings imply that transplants are predisposed to tolerance or rejection due to recipient developmental history and immunogenetic background.
Figures



Similar articles
-
Microchimerism: tolerance vs. sensitization.Curr Opin Organ Transplant. 2011 Aug;16(4):359-65. doi: 10.1097/MOT.0b013e3283484b57. Curr Opin Organ Transplant. 2011. PMID: 21666480 Free PMC article. Review.
-
Tolerance to noninherited maternal MHC antigens in mice.J Immunol. 2003 Nov 15;171(10):5554-61. doi: 10.4049/jimmunol.171.10.5554. J Immunol. 2003. PMID: 14607963
-
Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance.J Immunol. 2007 Nov 15;179(10):6749-61. doi: 10.4049/jimmunol.179.10.6749. J Immunol. 2007. PMID: 17982065
-
Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model.J Immunol. 2011 Feb 1;186(3):1442-9. doi: 10.4049/jimmunol.1003023. Epub 2010 Dec 22. J Immunol. 2011. PMID: 21178009 Free PMC article.
-
Tolerance to noninherited maternal antigens in mice and humans.Curr Opin Organ Transplant. 2009 Aug;14(4):439-47. doi: 10.1097/MOT.0b013e32832d6683. Curr Opin Organ Transplant. 2009. PMID: 19512930 Free PMC article. Review.
Cited by
-
Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice.J Clin Invest. 2011 Feb;121(2):582-92. doi: 10.1172/JCI44907. Epub 2011 Jan 18. J Clin Invest. 2011. PMID: 21245575 Free PMC article.
-
Exposure to non-inherited maternal antigens by breastfeeding affects antibody responsiveness.Haematologica. 2019 Feb;104(2):263-268. doi: 10.3324/haematol.2018.199406. Epub 2018 Sep 13. Haematologica. 2019. PMID: 30213833 Free PMC article.
-
Microchimerism: tolerance vs. sensitization.Curr Opin Organ Transplant. 2011 Aug;16(4):359-65. doi: 10.1097/MOT.0b013e3283484b57. Curr Opin Organ Transplant. 2011. PMID: 21666480 Free PMC article. Review.
-
Alterations in maternal-fetal cellular trafficking after fetal surgery.J Pediatr Surg. 2012 Jun;47(6):1089-94. doi: 10.1016/j.jpedsurg.2012.03.012. J Pediatr Surg. 2012. PMID: 22703775 Free PMC article.
-
Long-term feto-maternal microchimerism revisited: Microchimerism and tolerance in hematopoietic stem cell transplantation.Chimerism. 2010 Jul-Sep;1(1):39-43. doi: 10.4161/chim.1.1.12743. Chimerism. 2010. PMID: 21327150 Free PMC article.
References
-
- Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988 Sep 30;241(4874):1815–7. - PubMed
-
- Campbell DAJ, Lorber MI, Sweeton JC, Turcotte JG, Niederhuber JE, Beer AE. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation. 1984 Apr;37(4):340–4. - PubMed
-
- Opelz G. Analysis of the ‘NIMA effect’ in renal transplantation. Collaborative Transplant Study. Clin Transpl. 1990:63–7. - PubMed
-
- Cecka JM, Gjertson DW, Terasaki PI. Pediatric renal transplantation: a review of the UNOS data. United Network for Organ Sharing. Pediatr Transplant. 1997 Aug;1(1):55–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

