The YvfTU two-component system is involved in plcR expression in Bacillus cereus
- PMID: 18925929
- PMCID: PMC2588459
- DOI: 10.1186/1471-2180-8-183
The YvfTU two-component system is involved in plcR expression in Bacillus cereus
Abstract
Background: Most extracellular virulence factors produced by Bacillus cereus are regulated by the pleiotropic transcriptional activator PlcR. Among strains belonging to the B. cereus group, the plcR gene is always located in the vicinity of genes encoding the YvfTU two-component system. The putative role of YvfTU in the expression of the PlcR regulon was therefore investigated.
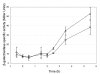
Results: Expression of the plcR gene was monitored using a transcriptional fusion with a lacZ reporter gene in a yvfTU mutant and in its B. cereus ATCC 14579 parental strain. Two hours after the onset of the stationary phase, a stage at which the PlcR regulon is highly expressed, the plcR expression in the yvfTU mutant was only 50% of that of its parental strain. In addition to the reduced plcR expression in the yvfTU mutant, a few members of the PlcR regulon showed a differential expression, as revealed by transcriptomic and proteomic analyses. The virulence of the yvfTU mutant in a Galleria mellonella insect model was slightly lower than that of the parental strain.
Conclusion: The YvfTU two-component system is not required for the expression of most of the virulence factors belonging to the PlcR regulon. However, YvfTU is involved in expression of plcR, a major regulator of virulence in B. cereus.
Figures



Similar articles
-
Specificity and polymorphism of the PlcR-PapR quorum-sensing system in the Bacillus cereus group.J Bacteriol. 2005 Feb;187(3):1182-7. doi: 10.1128/JB.187.3.1182-1187.2005. J Bacteriol. 2005. PMID: 15659693 Free PMC article.
-
The enhancin-like metalloprotease from the Bacillus cereus group is regulated by the pleiotropic transcriptional activator PlcR but is not essential for larvicidal activity.FEMS Microbiol Lett. 2006 Jul;260(1):9-16. doi: 10.1111/j.1574-6968.2006.00289.x. FEMS Microbiol Lett. 2006. PMID: 16790012
-
Characterization of a small PlcR-regulated gene co-expressed with cereolysin O.BMC Microbiol. 2007 Jun 7;7:52. doi: 10.1186/1471-2180-7-52. BMC Microbiol. 2007. PMID: 17555563 Free PMC article.
-
What sets Bacillus anthracis apart from other Bacillus species?Annu Rev Microbiol. 2009;63:451-76. doi: 10.1146/annurev.micro.091208.073255. Annu Rev Microbiol. 2009. PMID: 19514852 Review.
-
AtxA, a Bacillus anthracis global virulence regulator.Res Microbiol. 2010 Nov;161(9):735-42. doi: 10.1016/j.resmic.2010.09.006. Epub 2010 Sep 21. Res Microbiol. 2010. PMID: 20863885 Review.
Cited by
-
ResDE-dependent regulation of enterotoxin gene expression in Bacillus cereus: evidence for multiple modes of binding for ResD and interaction with Fnr.J Bacteriol. 2009 Jul;191(13):4419-26. doi: 10.1128/JB.00321-09. Epub 2009 Apr 24. J Bacteriol. 2009. PMID: 19395489 Free PMC article.
-
Identification of Bacillus cereus genes specifically expressed during growth at low temperatures.Appl Environ Microbiol. 2010 Apr;76(8):2562-73. doi: 10.1128/AEM.02348-09. Epub 2010 Feb 26. Appl Environ Microbiol. 2010. PMID: 20190083 Free PMC article.
-
Exposure of a 23F serotype strain of Streptococcus pneumoniae to cigarette smoke condensate is associated with selective upregulation of genes encoding the two-component regulatory system 11 (TCS11).Biomed Res Int. 2014;2014:976347. doi: 10.1155/2014/976347. Epub 2014 Jun 11. Biomed Res Int. 2014. PMID: 25013815 Free PMC article.
-
Bacterial quorum sensing: its role in virulence and possibilities for its control.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a012427. doi: 10.1101/cshperspect.a012427. Cold Spring Harb Perspect Med. 2012. PMID: 23125205 Free PMC article. Review.
-
The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects.J Bacteriol. 2009 Nov;191(22):7063-73. doi: 10.1128/JB.00892-09. Epub 2009 Sep 18. J Bacteriol. 2009. PMID: 19767427 Free PMC article.
References
-
- Agaisse H, Gominet M, Økstad OA, Kolstø AB, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol. 1999;32:1043–1053. - PubMed
-
- Gohar M, Økstad OA, Gilois N, Sanchis V, Kolstø AB, Lereclus D. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics. 2002;2:784–791. - PubMed
-
- Økstad OA, Gominet M, Purnelle B, Rose M, Lereclus D, Kolstø AB. Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology. 1999;145:3129–3138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

