Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis
- PMID: 18926825
- PMCID: PMC2796242
- DOI: 10.1053/j.gastro.2008.09.003
Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis
Abstract
Background & aims: Diabetic gastroparesis (delayed gastric emptying) is a well-recognized complication of diabetes that causes considerable morbidity and makes glucose control difficult. Interstitial cells of Cajal, which express the receptor tyrosine kinase Kit, are required for normal gastric emptying. We proposed that Kit expression is lost during diabetic gastroparesis due to increased levels of oxidative stress caused by low levels of heme oxygenase-1 (HO-1), an important cytoprotective molecule against oxidative injury.
Methods: Gastric emptying was measured in nonobese diabetic mice and correlated with levels of HO-1 expression and activity. Endogenous HO-1 activity was increased by administration of hemin and inhibited by chromium mesoporphyrin.
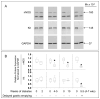
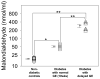
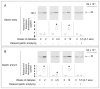
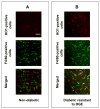
Results: In early stages of diabetes, HO-1 was up-regulated in gastric macrophages and remained up-regulated in all mice that were resistant to development of delayed gastric emptying. In contrast, HO-1 did not remain up-regulated in all the mice that developed delayed gastric emptying; expression of Kit and neuronal nitric oxide synthase decreased markedly in these mice. Loss of HO-1 up-regulation increased levels of reactive oxygen species. Induction of HO-1 by hemin decreased reactive oxygen species, rapidly restored Kit and neuronal nitric oxide synthase expression, and completely normalized gastric emptying in all mice. Inhibition of HO-1 activity in mice with normal gastric emptying caused a loss of Kit expression and development of diabetic gastroparesis.
Conclusions: Induction of the HO-1 pathway prevents and reverses cellular changes that lead to development of gastrointestinal complications of diabetes. Reagents that induce this pathway might therefore be developed as therapeutics.
Conflict of interest statement
Conflict of Interest: None
Figures




References
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–81. - PubMed
-
- Wardle EN. How does hyperglycaemia predispose to diabetic nephropathy? Qjm. 1996;89:943–51. - PubMed
-
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–9. - PubMed
-
- Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–6. - PubMed
-
- Horvath VJ, Vittal H, Ordog T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

