Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin
- PMID: 18926829
- PMCID: PMC2630487
- DOI: 10.1016/j.yjmcc.2008.08.015
Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin
Abstract
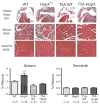
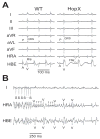
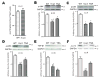
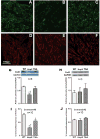
Atrial fibrosis influences the development of atrial fibrillation (AF), particularly in the setting of structural heart disease where angiotensin-inhibition is partially effective for reducing atrial fibrosis and AF. Histone-deacetylase inhibition reduces cardiac hypertrophy and fibrosis, so we sought to determine if the HDAC inhibitor trichostatin A (TSA) could reduce atrial fibrosis and arrhythmias. Mice over-expressing homeodomain-only protein (HopX(Tg)), which recruits HDAC activity to induce cardiac hypertrophy were investigated in 4 groups (aged 14-18 weeks): wild-type (WT), HopX(Tg), HopX(Tg) mice treated with TSA for 2 weeks (TSA-HopX) and wild-type mice treated with TSA for 2 weeks (TSA-WT). These groups were characterized using invasive electrophysiology, atrial fibrosis measurements, atrial connexin immunocytochemistry and myocardial angiotensin II measurements. Invasive electrophysiologic stimulation, using the same attempts in each group, induced more atrial arrhythmias in HopX(Tg) mice (48 episodes in 13 of 15 HopX(Tg) mice versus 5 episodes in 2 of 15 TSA-HopX mice, P<0.001; versus 9 episodes in 2 of 15 WT mice, P<0.001; versus no episodes in any TSA-WT mice, P<0.001). TSA reduced atrial arrhythmia duration in HopX(Tg) mice (1307+/-289 ms versus 148+/-110 ms, P<0.01) and atrial fibrosis (8.1+/-1.5% versus 3.9+/-0.4%, P<0.001). Atrial connexin40 was lower in HopX(Tg) compared to WT mice, and TSA normalized the expression and size distribution of connexin40 gap junctions. Myocardial angiotensin II levels were similar between WT and HopX(Tg) mice (76.3+/-26.0 versus 69.7+/-16.6 pg/mg protein, P=NS). Therefore, it appears HDAC-inhibition reverses atrial fibrosis, connexin40 remodeling and atrial arrhythmia vulnerability independent of angiotensin II in cardiac hypertrophy.
Figures
Comment in
-
Insights into mechanisms linking cardiac hypertrophy and atrial fibrosis: evidence for a role of histone deacetylase in atrial fibrillation pathophysiology and therapy.J Mol Cell Cardiol. 2008 Dec;45(6):707-8. doi: 10.1016/j.yjmcc.2008.09.001. Epub 2008 Sep 10. J Mol Cell Cardiol. 2008. PMID: 18817781 No abstract available.
References
-
- Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–44. - PubMed
-
- Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset Atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–8. - PubMed
-
- Rotter M, Jais P, Garrigue S, Sanders P, Hocini M, Hsu L-F, et al. Clinical predictors of noninducibility of sustained atrial fibrillation after pulmonary vein isolation. J Cardiovas Electrophysiol. 2005;16:1298–303. - PubMed
-
- Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. - PubMed
-
- Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

