Luminal flow patterns dictate arterial drug deposition in stent-based delivery
- PMID: 18926864
- PMCID: PMC2836846
- DOI: 10.1016/j.jconrel.2008.09.075
Luminal flow patterns dictate arterial drug deposition in stent-based delivery
Erratum in
- J Control Release. 2010 Aug 17;146(1):160
Abstract
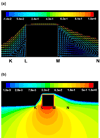
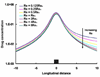
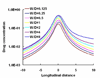
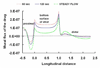
Endovascular stents reside in a dynamic flow environment and yet the impact of flow on arterial drug deposition after stent-based delivery is only now emerging. We employed computational fluid dynamic modeling tools to investigate the influence of luminal flow patterns on arterial drug deposition and distribution. Flow imposes recirculation zones distal and proximal to the stent strut that extend the coverage of tissue absorption of eluted drug and induce asymmetry in tissue drug distribution. Our analysis now explains how the disparity in sizes of the two recirculation zones and the asymmetry in drug distribution are determined by a complex interplay of local flow and strut geometry. When temporal periodicity was introduced as a model of pulsatile flow, the net luminal flow served as an index of flow-mediated spatio-temporal tissue drug uptake. Dynamically changing luminal flow patterns are intrinsic to the coronary arterial tree. Coronary drug-eluting stents should be appropriately considered where luminal flow, strut design and pulsatility have direct effects on tissue drug uptake after local delivery.
Figures






Comment in
-
Better design of drug-eluting stents using computer modeling.J Control Release. 2009 Jan 5;133(1):1. doi: 10.1016/j.jconrel.2008.11.005. Epub 2008 Nov 20. J Control Release. 2009. PMID: 19038297 No abstract available.
References
-
- Hwang CW, Wu D, Edelman ER. Physiological transport forces govern drug distribution for stent-based delivery. Circulation. 2001;104(5):600–605. - PubMed
-
- Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. - PubMed
-
- Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112(2):270–278. - PubMed
-
- Finkelstein A, McClean D, Kar S, Takizawa K, Varghese K, Baek N, Park K, Fishbein MC, Makkar R, Litvack F, Eigler NL. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003;107(5):777–784. - PubMed
-
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

