Decomposing health: tolerance and resistance to parasites in animals
- PMID: 18926971
- PMCID: PMC2666700
- DOI: 10.1098/rstb.2008.0184
Decomposing health: tolerance and resistance to parasites in animals
Abstract
Plant biologists have long recognized that host defence against parasites and pathogens can be divided into two conceptually different components: the ability to limit parasite burden (resistance) and the ability to limit the harm caused by a given burden (tolerance). Together these two components determine how well a host is protected against the effects of parasitism. This distinction is useful because it recognizes that hosts that are best at controlling parasite burdens are not necessarily the healthiest. Moreover, resistance and tolerance can be expected to have different effects on the epidemiology of infectious diseases and host-parasite coevolution. However, studies of defence in animals have to date focused on resistance, whereas the possibility of tolerance and its implications have been largely overlooked. The aim of our review is to (i) describe the statistical framework for analysis of tolerance developed in plant science and how this can be applied to animals, (ii) review evidence of genetic and environmental variation for tolerance in animals, and studies indicating which mechanisms could contribute to this variation, and (iii) outline avenues for future research on this topic.
Figures
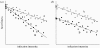
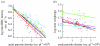


References
-
- Aidoo M., Terlouw D.J., Kolczak M., McElroy P.D., ter Kuile F.O., Kariuki S., Nahlen B.L., Lal A.A., Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi:10.1016/S0140-6736(02)08273-9 - DOI - PubMed
-
- Aliberti J., Serhan C., Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 2002;196:1253–1262. doi:10.1084/jem.20021183 - DOI - PMC - PubMed
-
- Allen S.J., O'Donnell A., Alexander N.D.E., Alpers M.P., Peto T.E.A., Clegg J.B., Weatherall D.J. α+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc. Natl Acad. Sci. USA. 1997;94:14 736–14 741. doi:10.1073/pnas.94.26.14736 - DOI - PMC - PubMed
-
- Ayres J., Freitag N., Schneider D. Identification of Drosophila mutants altering defense to and endurance of Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi:10.1534/genetics.107.083782 - DOI - PMC - PubMed
-
- Bisset S.A., Morris C.A. Feasibility and implications of breeding sheep for resilience to nematode challenge. Int. J. Parasitol. 1996;26:857–868. doi:10.1016/S0020-7519(96)80056-7 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
