Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling
- PMID: 18927075
- PMCID: PMC2596378
- DOI: 10.1074/jbc.M807029200
Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling
Abstract
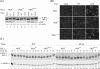
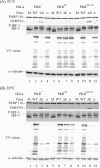
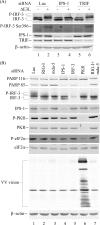
Interferon regulatory factor 3 (IRF-3) undergoes phosphorylation-induced activation in virus-infected cells and plays an important role in the antiviral innate immune response. The E3L protein encoded by vaccinia virus is known to impair phosphorylation and activation of IRF-3. Kinases in addition to I kappaB kinase-related kinases are implicated in the IRF-3-dependent antiviral response. To test in human cells the role of the protein kinase regulated by RNA (PKR) in IRF-3 activation, HeLa cells made stably deficient in PKR using an RNA interference strategy were compared with PKR-sufficient cells. Rapid phosphorylation and nuclear accumulation of IRF-3 were detected in PKR-sufficient cells following infection with E3L deletion mutant (DeltaE3L) virus. By contrast, the full IRF-3 activation response was largely abolished in PKR-deficient cells. The DeltaE3L virus-induced IRF-3 activation seen in PKR-sufficient cells was diminished by treatment with cytosine beta-D-arabinofuranoside. Furthermore, the vaccinia mutant ts23, which displays increased viral double-stranded RNA production at 39 degrees C, induced PKR-dependent IRF-3 phosphorylation at 39 degrees C but not at 31 degrees C. Both IRF-3 phosphorylation and cell apoptosis induced by infection with DeltaE3L virus were dependent upon RIG-I-like receptor signal transduction components, including the adapter IPS-1. These data suggest that PKR facilitates the host innate immune response and apoptosis in virus-infected cells by mediating IRF-3 activation through the mitochondrial IPS-1 signal transduction pathway.
Figures


Similar articles
-
Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L.J Virol. 2009 Jun;83(11):5718-25. doi: 10.1128/JVI.00224-09. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321614 Free PMC article.
-
Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis.J Virol. 2008 Jan;82(2):840-8. doi: 10.1128/JVI.01891-07. Epub 2007 Oct 24. J Virol. 2008. PMID: 17959656 Free PMC article.
-
IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by Vaccinia virus E3L protein.J Biol Chem. 2001 Mar 23;276(12):8951-7. doi: 10.1074/jbc.M008717200. Epub 2000 Dec 21. J Biol Chem. 2001. PMID: 11124948
-
The dsRNA protein kinase PKR: virus and cell control.Biochimie. 2007 Jun-Jul;89(6-7):799-811. doi: 10.1016/j.biochi.2007.03.001. Epub 2007 Mar 12. Biochimie. 2007. PMID: 17451862 Review.
-
Vaccinia virus recombinants as a model system to analyze interferon-induced pathways.J Interferon Cytokine Res. 2004 Nov;24(11):637-46. doi: 10.1089/jir.2004.24.637. J Interferon Cytokine Res. 2004. PMID: 15684816 Review.
Cited by
-
Pathogen recognition and inflammatory signaling in innate immune defenses.Clin Microbiol Rev. 2009 Apr;22(2):240-73, Table of Contents. doi: 10.1128/CMR.00046-08. Clin Microbiol Rev. 2009. PMID: 19366914 Free PMC article. Review.
-
Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity.J Virol. 2009 Mar;83(5):2298-309. doi: 10.1128/JVI.01245-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109397 Free PMC article.
-
Protein synthesis inhibition and GADD34 control IFN-β heterogeneous expression in response to dsRNA.EMBO J. 2017 Mar 15;36(6):761-782. doi: 10.15252/embj.201695000. Epub 2017 Jan 18. EMBO J. 2017. PMID: 28100675 Free PMC article.
-
HSV-1 Triggers an Antiviral Transcriptional Response during Viral Replication That Is Completely Abrogated in PKR-/- Cells.Pathogens. 2023 Sep 3;12(9):1126. doi: 10.3390/pathogens12091126. Pathogens. 2023. PMID: 37764935 Free PMC article.
-
How Influenza A Virus NS1 Deals with the Ubiquitin System to Evade Innate Immunity.Viruses. 2021 Nov 19;13(11):2309. doi: 10.3390/v13112309. Viruses. 2021. PMID: 34835115 Free PMC article. Review.
References
-
- Akira, S., and Hemmi, H. (2003) Immunol. Lett. 85 85–95 - PubMed
-
- Yoneyama, M., and Fujita, T. (2007) J. Biol. Chem. 282 15315–15318 - PubMed
-
- Onomoto, K., Yoneyama, M., and Fujita, T. (2007) Curr. Top. Microbiol. Immunol. 316 193–205 - PubMed
-
- Le Bon, A., and Tough, D. F. (2002) Curr. Opin. Immunol. 14 432–436 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

