Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy
- PMID: 18927082
- PMCID: PMC3259888
- DOI: 10.1074/jbc.M806353200
Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy
Abstract
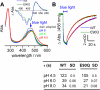
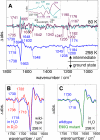
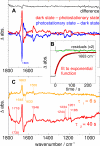
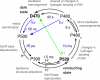
Channelrhodopsin-2 (ChR2) is a microbial type rhodopsin and a light-gated cation channel that controls phototaxis in Chlamydomonas. We expressed ChR2 in COS-cells, purified it, and subsequently investigated this unusual photoreceptor by flash photolysis and UV-visible and Fourier transform infrared difference spectroscopy. Several transient photoproducts of the wild type ChR2 were identified, and their kinetics and molecular properties were compared with those of the ChR2 mutant E90Q. Based on the spectroscopic data we developed a model of the photocycle comprising six distinguishable intermediates. This photocycle shows similarities to the photocycle of the ChR2-related Channelrhodopsin of Volvox but also displays significant differences. We show that molecular changes include retinal isomerization, changes in hydrogen bonding of carboxylic acids, and large alterations of the protein backbone structure. These alterations are stronger than those observed in the photocycle of other microbial rhodopsins like bacteriorhodopsin and are related to those occurring in animal rhodopsins. UV-visible and Fourier transform infrared difference spectroscopy revealed two late intermediates with different time constants of tau = 6 and 40 s that exist during the recovery of the dark state. The carboxylic side chain of Glu(90) is involved in the slow transition. The molecular changes during the ChR2 photocycle are discussed with respect to other members of the rhodopsin family.
Figures

References
-
- Nagel, G., Ollig, D., Fuhrmann, M., Kateriya, S., Musti, A. M., Bamberg, E., and Hegemann, P. (2002) Science 296 2395-2398 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

