Mammalian phosphomannomutase PMM1 is the brain IMP-sensitive glucose-1,6-bisphosphatase
- PMID: 18927083
- PMCID: PMC2662221
- DOI: 10.1074/jbc.M805224200
Mammalian phosphomannomutase PMM1 is the brain IMP-sensitive glucose-1,6-bisphosphatase
Abstract
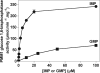
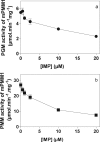
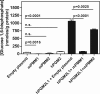
Glucose 1,6-bisphosphate (Glc-1,6-P(2)) concentration in brain is much higher than what is required for the functioning of phosphoglucomutase, suggesting that this compound has a role other than as a cofactor of phosphomutases. In cell-free systems, Glc-1,6-P(2) is formed from 1,3-bisphosphoglycerate and Glc-6-P by two related enzymes: PGM2L1 (phosphoglucomutase 2-like 1) and, to a lesser extent, PGM2 (phosphoglucomutase 2). It is hydrolyzed by the IMP-stimulated brain Glc-1,6-bisphosphatase of still unknown identity. Our aim was to test whether Glc-1,6-bisphosphatase corresponds to the phosphomannomutase PMM1, an enzyme of mysterious physiological function sharing several properties with Glc-1,6-bisphosphatase. We show that IMP, but not other nucleotides, stimulated by >100-fold (K(a) approximately 20 mum) the intrinsic Glc-1,6-bisphosphatase activity of recombinant PMM1 while inhibiting its phosphoglucomutase activity. No such effects were observed with PMM2, an enzyme paralogous to PMM1 that physiologically acts as a phosphomannomutase in mammals. Transfection of HEK293T cells with PGM2L1, but not the related enzyme PGM2, caused an approximately 20-fold increase in the concentration of Glc-1,6-P(2). Transfection with PMM1 caused a profound decrease (>5-fold) in Glc-1,6-P(2) in cells that were or were not cotransfected with PGM2L1. Furthermore, the concentration of Glc-1,6-P(2) in wild-type mouse brain decreased with time after ischemia, whereas it did not change in PMM1-deficient mouse brain. Taken together, these data show that PMM1 corresponds to the IMP-stimulated Glc-1,6-bisphosphatase and that this enzyme is responsible for the degradation of Glc-1,6-P(2) in brain. In addition, the role of PGM2L1 as the enzyme responsible for the synthesis of the elevated concentrations of Glc-1,6-P(2) in brain is established.
Figures
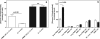
References
-
- Leloir, L. F., Trucco, R. E., Cardini, C. E., Paladini, A. C., and Caputto, R. (1948) Arch. Biochem. 19 339–340 - PubMed
-
- Passonneau, J. V., Lowry, O. H., Schulz, D. W., and Brown, J. G. (1969) J. Biol. Chem. 244 902–909 - PubMed
-
- Krzanowski, J., and Matschinsky, F. M. (1969) Biochem. Biophys. Res. Commun. 34 816–823 - PubMed
-
- Rose, I. A., and Warms, J. V. B. (1974) Biochem. Biophys. Res. Commun. 59 1333–1340 - PubMed
-
- Koster, J. F., Slee, R. G., Staal, G. E., and van Berkel, T. J. (1972) Biochim. Biophys. Acta 258 763–768 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

