Preparation and biological evaluation of 64Cu-CB-TE2A-sst2-ANT, a somatostatin antagonist for PET imaging of somatostatin receptor-positive tumors
- PMID: 18927338
- PMCID: PMC2794832
- DOI: 10.2967/jnumed.108.054502
Preparation and biological evaluation of 64Cu-CB-TE2A-sst2-ANT, a somatostatin antagonist for PET imaging of somatostatin receptor-positive tumors
Abstract
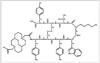
Recently, the somatostatin receptor subtype 2 (SSTR2) selective antagonist sst2-ANT was determined to have a high affinity for SSTR2. Additionally, 111In-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-sst2-ANT showed high uptake in an SSTR2-transfected, tumor-bearing mouse model and suggested that radiolabeled SSTR2 antagonists may be superior to agonists for imaging SSTR2-positive tumors. This report describes the synthesis and evaluation of 64Cu-CB-4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-sst2-ANT (64Cu-CB-TE2A-sst2-ANT) as a PET radiopharmaceutical for the in vivo imaging of SSTR2-positive tumors.
Methods: Receptor-binding studies were performed to determine the dissociation constant of the radiopharmaceutical 64Cu-CB-TE2A-sst2-ANT using AR42J rat pancreatic tumor cell membranes. The internalization of 64Cu-CB-TE2A-sst2-ANT was compared with that of the 64Cu-labeled agonist 64Cu-CB-TE2A-tyrosine3-octreotate (64Cu-CB-TE2A-Y3-TATE) in AR42J cells. Both radiopharmaceuticals were also compared in vivo through biodistribution studies using healthy rats bearing AR42J tumors, and small-animal PET/CT of 64Cu-CB-TE2A-sst2-ANT was performed.
Results: The dissociation constant value for the radiopharmaceutical was determined to be 26 +/- 2.4 nM, and the maximum number of binding sites was 23,000 fmol/mg. 64Cu-CB-TE2A-sst2-ANT showed significantly less internalization than did 64Cu-CB-TE2A-Y3-TATE at time points from 15 min to 4 h. Biodistribution studies revealed that the clearance of 64Cu-CB-TE2A-sst2-ANT from the blood was rapid, whereas the clearance of 64Cu-CB-TE2A-sst2-ANT from the liver and kidneys was more modest at all time points. Tumor-to-blood and tumor-to-muscle ratios were determined to be better for 64Cu-CB-TE2A-sst2-ANT than those for 64Cu-CB-TE2A-Y3-TATE at the later time points, although liver and kidney uptake was significantly higher. Small-animal imaging using 64Cu-CB-TE2A-sst2-ANT revealed excellent tumor-to-background contrast at 4 h after injection, and standardized uptake values remained high even after 24 h.
Conclusion: The PET radiopharmaceutical 64Cu-CB-TE2A-sst2-ANT is an attractive agent, worthy of future study as a PET radiopharmaceutical for the imaging of somatostatin receptor-positive tumors.
Figures





References
-
- Rogers BE, McLean SF, Kirkman RL, et al. In vivo localization of [111In]-DTPA-d-Phe1-octreotide to human ovarian tumor xenografts induced to express the somatostatin receptor subtype 2 using an adenoviral vector. Clin Cancer Res. 1999;5:383–393. - PubMed
-
- Anderson CJ, Dehdashti F, Cutler PD, et al. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–221. - PubMed
-
- Li WP, Meyer LA, Anderson CJ. Radiopharmaceuticals for positron emission tomography imaging of somatostatin receptor positive tumors. Top Curr Chem. 2005;252:179–192.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
