Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress
- PMID: 18927433
- PMCID: PMC2644087
- DOI: 10.1182/blood-2008-05-158584
Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress
Abstract
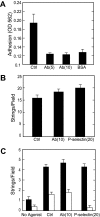
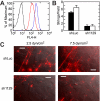
Acutely secreted von Willebrand factor (VWF) multimers adhere to endothelial cells, support platelet adhesion, and may induce microvascular thrombosis. Immunofluorescence microscopy of live human umbilical vein endothelial cells showed that VWF multimers rapidly formed strings several hundred micrometers long on the cell surface after stimulation with histamine. Unexpectedly, only a subset of VWF strings supported platelet binding, which depended on platelet glycoprotein Ib. Electron microscopy showed that VWF strings often consisted of bundles and networks of VWF multimers, and each string was tethered to the cell surface by a limited number of sites. Several approaches implicated P-selectin and integrin alpha(v)beta(3) in anchoring VWF strings. An RGDS peptide or a function-blocking antibody to integrin alpha(v)beta(3) reduced the number of VWF strings formed. In addition, integrin alpha(v) decorated the VWF strings by immunofluorescence microscopy. Furthermore, lentiviral transduction of shRNA against the alpha(v) subunit reduced the expression of cell-surface integrin alpha(v)beta(3) and impaired the ability of endothelial cells to retain VWF strings. Soluble P-selectin reduced the number of platelet-decorated VWF strings in the absence of Ca(2+) and Mg(2+) but had no effect in the presence of these cations. These results indicate that VWF strings bind specifically to integrin alpha(v)beta(3) on human endothelial cells.
Figures
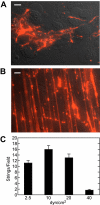
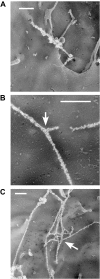
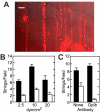
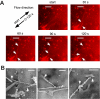


Comment in
-
Fishing for platelets.Blood. 2009 Feb 12;113(7):1397-8. doi: 10.1182/blood-2008-11-188524. Blood. 2009. PMID: 19221043
References
-
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. - PubMed
-
- Sadler JE. von Willebrand factor: two sides of a coin. J Thromb Haemost. 2005;3:1702–1709. - PubMed
-
- Furlan M, Robles R, Lamie B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. - PubMed
-
- Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

