Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells
- PMID: 18927477
- PMCID: PMC2729695
- DOI: 10.1634/stemcells.2008-0439
Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells
Abstract
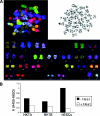
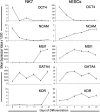
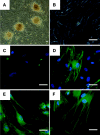
Several reports have documented the derivation of pluripotent cells (multipotent germline stem cells) from spermatogonial stem cells obtained from the adult mouse testis. These spermatogonia-derived stem cells express embryonic stem cell markers and differentiate to the three primary germ layers, as well as the germline. Data indicate that derivation may involve reprogramming of endogenous spermatogonia in culture. Here, we report the derivation of human multipotent germline stem cells (hMGSCs) from a testis biopsy. The cells express distinct markers of pluripotency, form embryoid bodies that contain derivatives of all three germ layers, maintain a normal XY karyotype, are hypomethylated at the H19 locus, and express high levels of telomerase. Teratoma assays indicate the presence of human cells 8 weeks post-transplantation but limited teratoma formation. Thus, these data suggest the potential to derive pluripotent cells from human testis biopsies but indicate a need for novel strategies to optimize hMGSC culture conditions and reprogramming.
Figures



References
-
- Kluin PM, de Rooij DG. A comparison between the morphology and cell kinetics of gonocytes and adult type undifferentiated spermatogonia in the mouse. Int J Androl. 1981;4:475–493. - PubMed
-
- Rossant J. Stem cells from the mammalian blastocyst. Stem Cells. 2001;19:477–482. - PubMed
-
- Evans M, Hunter S. Source and nature of embryonic stem cells. C R Biol. 2002;325:1003–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

