DeltaNp63 is essential for epidermal commitment of embryonic stem cells
- PMID: 18927616
- PMCID: PMC2562986
- DOI: 10.1371/journal.pone.0003441
DeltaNp63 is essential for epidermal commitment of embryonic stem cells
Abstract
In vivo studies have demonstrated that p63 plays complex and pivotal roles in pluristratified squamous epithelial development, but its precise function and the nature of the isoform involved remain controversial. Here, we investigate the role of p63 in epithelial differentiation, using an in vitro ES cell model that mimics the early embryonic steps of epidermal development. We show that the DeltaNp63 isoform is activated soon after treatment with BMP-4, a morphogen required to commit differentiating ES cells from a neuroectodermal to an ectodermal cell fate. DeltaNp63 gene expression remains high during epithelial development. P63 loss of function drastically prevents ectodermal cells to commit to the K5/K14-positive stratified epithelial pathway while gain of function experiments show that DeltaNp63 allows this commitment. Interestingly, other epithelial cell fates are not affected, allowing the production of K5/K18-positive epithelial cells. Therefore, our results demonstrate that DeltaNp63 may be dispensable for some epithelial differentiation, but is necessary for the commitment of ES cells into K5/K14-positive squamous stratified epithelial cells.
Conflict of interest statement
Figures

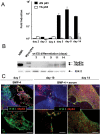

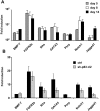

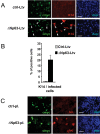
References
-
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. - PubMed
-
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. - PubMed
-
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. - PubMed
-
- Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

