Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial
- PMID: 18928378
- PMCID: PMC2665183
- DOI: 10.1086/593214
Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial
Abstract
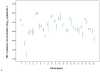
A randomized cross-over trial of herpes simplex virus type 2 (HSV-2)-suppressive therapy (valacyclovir, 500 mg twice daily, or placebo for 8 weeks, a 2-week washout period, then the alternative therapy for 8 weeks) was conducted among 20 Peruvian women coinfected with HSV-2 and human immunodeficiency virus type 1 (HIV-1) who were not on antiretroviral therapy. Plasma samples (obtained weekly) and endocervical swab specimens (obtained thrice weekly) were collected for HIV-1 RNA polymerase chain reaction. Plasma HIV-1 level was significantly lower during the valacyclovir arm, compared with the placebo arm (-0.26 log10 copies/mL, a 45% decrease [P < .001]), as was cervical HIV-1 level (-0.35 log10 copies/swab, a 55% decrease [P < .001]). Suppressive HSV-2 therapy has the potential to reduce HIV-1 infectiousness and slow HIV-1 disease progression.
Trial registration: ClinicalTrials.gov NCT00465205.
Conflict of interest statement
Potential conflicts of interest: C.C. has received research grant support from the GlaxoSmithKline (GSK) and has served on an advisory board for GSK. J.S. has received grant support from GSK. A.W. has received grant support GSK, Antigenics, 3M, Roche, and Vical; she is a consultant for Novartis, PowderMed, and MediGene and is a speaker for Merck Vaccines. The University of Washington Virology Division Laboratories have received grant funding from GSK and Novartis to perform herpes simplex virus serologic assays and polymerase chain reaction assays for studies funded by these companies. L.C. directs these laboratories. He receives no salary support from these grants.
Figures

Comment in
-
Time to refocus on HSV interventions for HIV prevention?J Infect Dis. 2011 Dec 15;204(12):1822-6. doi: 10.1093/infdis/jir653. Epub 2011 Oct 12. J Infect Dis. 2011. PMID: 21998480 Free PMC article. No abstract available.
References
-
- Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. - PubMed
-
- Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189:1466–71. - PubMed
-
- Schacker T, Hu HL, Koelle DM, et al. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:21–8. - PubMed
-
- Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. - PubMed
-
- Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. - PubMed

