Stabilized collagen scaffolds for heart valve tissue engineering
- PMID: 18928400
- PMCID: PMC2792094
- DOI: 10.1089/ten.tea.2008.0263
Stabilized collagen scaffolds for heart valve tissue engineering
Abstract
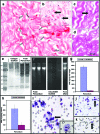
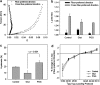
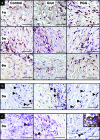
Scaffolds for heart valve tissue engineering must function immediately after implantation but also need to tolerate cell infiltration and gradual remodeling. We hypothesized that moderately cross-linked collagen scaffolds would fulfill these requirements. To test our hypothesis, scaffolds prepared from decellularized porcine pericardium were treated with penta-galloyl glucose (PGG), a collagen-binding polyphenol, and tested for biodegradation, biaxial mechanical properties, and in vivo biocompatibility. For controls, we used un-cross-linked scaffolds and glutaraldehyde-treated scaffolds. Results confirmed complete pericardium decellularization and the ability of scaffolds to encourage fibroblast chemotaxis and to aid in creation of anatomically correct valve-shaped constructs. Glutaraldehyde cross-linking fully stabilized collagen but did not allow for tissue remodeling and calcified when implanted subdermally in rats. PGG-treated collagen was initially resistant to collagenase and then degraded gradually, indicating partial stabilization. Moreover, PGG-treated pericardium exhibited excellent biaxial mechanical properties, did not calcify in vivo, and supported infiltration by host fibroblasts and subsequent matrix remodeling. In conclusion, PGG-treated acellular pericardium is a promising scaffold for heart valve tissue engineering.
Figures



Similar articles
-
Stabilized Collagen and Elastin-Based Scaffolds for Mitral Valve Tissue Engineering.Tissue Eng Part A. 2016 Nov;22(21-22):1241-1251. doi: 10.1089/ten.TEA.2016.0032. Epub 2016 Oct 3. Tissue Eng Part A. 2016. PMID: 27608885 Free PMC article.
-
Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility.Tissue Eng Part C Methods. 2010 Aug;16(4):671-83. doi: 10.1089/ten.TEC.2009.0111. Tissue Eng Part C Methods. 2010. PMID: 19780649
-
Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol.Biomaterials. 2013 Jan;34(3):685-95. doi: 10.1016/j.biomaterials.2012.09.081. Epub 2012 Oct 24. Biomaterials. 2013. PMID: 23103157 Free PMC article.
-
The extracellular matrix as a biologic scaffold material.Biomaterials. 2007 Sep;28(25):3587-93. doi: 10.1016/j.biomaterials.2007.04.043. Epub 2007 May 8. Biomaterials. 2007. PMID: 17524477 Review.
-
Marine collagen scaffolds in tissue engineering.Curr Opin Biotechnol. 2022 Apr;74:92-103. doi: 10.1016/j.copbio.2021.10.011. Epub 2021 Dec 14. Curr Opin Biotechnol. 2022. PMID: 34920212 Review.
Cited by
-
Stabilized Collagen and Elastin-Based Scaffolds for Mitral Valve Tissue Engineering.Tissue Eng Part A. 2016 Nov;22(21-22):1241-1251. doi: 10.1089/ten.TEA.2016.0032. Epub 2016 Oct 3. Tissue Eng Part A. 2016. PMID: 27608885 Free PMC article.
-
Development and testing of a transcatheter heart valve with reduced calcification potential.Front Cardiovasc Med. 2023 Dec 6;10:1270496. doi: 10.3389/fcvm.2023.1270496. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38124891 Free PMC article.
-
Heart valve tissue-derived hydrogels: Preparation and characterization of mitral valve chordae, aortic valve, and mitral valve gels.J Biomed Mater Res B Appl Biomater. 2019 Jul;107(5):1732-1740. doi: 10.1002/jbm.b.34266. Epub 2018 Nov 12. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30419146 Free PMC article.
-
Pentagalloyl Glucose and Its Functional Role in Vascular Health: Biomechanics and Drug-Delivery Characteristics.Ann Biomed Eng. 2019 Jan;47(1):39-59. doi: 10.1007/s10439-018-02145-5. Epub 2018 Oct 8. Ann Biomed Eng. 2019. PMID: 30298373 Free PMC article. Review.
-
Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure.Biomaterials. 2018 Jan;150:25-37. doi: 10.1016/j.biomaterials.2017.10.011. Epub 2017 Oct 6. Biomaterials. 2018. PMID: 29031049 Free PMC article.
References
-
- Yacoub M. Viewpoint: heart valve engineering. Interview by James Butcher. Circulation. 2007;116:f44. - PubMed
-
- Schoen FJ. New frontiers in the pathology and therapy of heart valve disease: 2006 Society for Cardiovascular Pathology, Distinguished Achievement Award Lecture, United States-Canadian Academy of Pathology, Atlanta, GA, February 12, 2006. Cardiovasc Pathol. 2006;15:271. - PubMed
-
- Simionescu D. Simionescu A. Deac R. Mapping of glutaraldehyde-treated bovine pericardium and tissue selection for bioprosthetic heart valves. J Biomed Mater Res. 1993;27:697. - PubMed
-
- Simionescu D. Iozzo RV. Kefalides NA. Bovine pericardial proteoglycan: biochemical, immunochemical and ultrastructural studies. Matrix. 1989;9:301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

