PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility
- PMID: 18929570
- PMCID: PMC2752880
- DOI: 10.1016/j.yjmcc.2008.09.124
PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility
Abstract
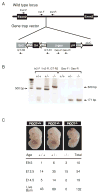
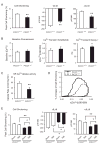
PICOT (PKC-interacting cousin of thioredoxin) was previously shown to inhibit the development of cardiac hypertrophy, concomitant with an increase in cardiomyocyte contractility. To explore the physiological function of PICOT in the hearts, we generated a PICOT-deficient mouse line by using a gene trap approach. PICOT(-/-) mice were embryonic lethal indicating that PICOT plays an essential role during embryogenesis, whereas PICOT(+/-) mice were viable with no apparent morphological defects. The PICOT protein levels were reduced by about 50% in the hearts of PICOT(+/-) mice. Significantly exacerbated cardiac hypertrophy was induced by pressure overload in PICOT(+/-) mice relative to that seen in wild type littermates. In line with this observation, calcineurin-NFAT signaling was greatly enhanced by pressure overload in the hearts of PICOT(+/-) mice. Cardiomyocytes from PICOT(+/-) mice exhibited significantly reduced contractility, which may be due in part to hypophosphorylation of phospholamban and reduced SERCA activity. These data indicate that the precise PICOT protein level significantly affects the process of cardiac hypertrophy and cardiomyocyte contractility. We suggest that PICOT plays as a critical negative regulator of cardiac hypertrophy and a positive inotropic regulator.
Figures



References
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews. 2006 Aug;7(8):589–600. - PubMed
-
- Zimmer HG, Gerdes AM, Lortet S, Mall G. Changes in heart function and cardiac cell size in rats with chronic myocardial infarction. Journal of molecular and cellular cardiology. 1990 Nov;22(11):1231–43. - PubMed
-
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 1991 Sep 15;88(18):8277–81. - PMC - PubMed
-
- Lips DJ, deWindt LJ, van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. European heart journal. 2003 May;24(10):883–96. - PubMed
-
- Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? American journal of physiology. 2005 Jul;289(1):H8–H16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

