Delayed rejection of MHC class II-disparate skin allografts in mice treated with farnesyltransferase inhibitors
- PMID: 18930822
- PMCID: PMC7369389
- DOI: 10.1016/j.trim.2008.09.011
Delayed rejection of MHC class II-disparate skin allografts in mice treated with farnesyltransferase inhibitors
Abstract
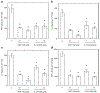
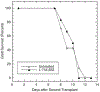
Farnesyltransferase inhibitors (FTIs), developed as anti-cancer drugs, have the potential to modulate immune responses without causing nonspecific immune suppression. We have investigated the possibility that FTIs, by affecting T cell cytokine secretion, can attenuate alloreactive immune responses. The effects of FTIs on murine alloreactive T cells were determined both in vitro, by measuring cytokine secretion or cell proliferation in mixed lymphocyte cultures, and in vivo, by performing skin allografts from H-2(bm12) mice to MHC class II-disparate B6 mice. We found that two different FTIs, ABT-100 and L-744,832, blocked secretion of IFN-gamma, IL-2, IL-4, and TNF-alpha from naïve T cells in vitro. ABT-100 and L-744,832 blocked cytokine production from both CD4(+) and CD8(+) naïve T cells stimulated with CD3 and CD28 antibodies, but only if the cells were pretreated with the FTIs for 48 h. Proliferation of alloreactive T cells in mixed lymphocyte cultures was blocked by either FTI. We also found that the proliferation of enriched T cells stimulated with IL-2 was blocked by ABT-100 treatment. In mice with an MHC class II-disparate skin graft, rejection of primary allografts was significantly delayed by treatment with either ABT-100 or L-744,832. Secondary rejection in mice previously primed to the alloantigen was found to be unaffected by L-744,832 treatment. We have shown that FTIs can block T cell cytokine secretion and attenuate alloreactive immune responses. The ability of FTIs to block secretion of cytokines, including IFN-gamma and IL-4, from naïve T cells provides a likely biological mechanism for the specific suppression of class II MHC-mediated allorejection. These results demonstrate that FTIs may have useful immunomodulatory activity due to their ability to delay priming to alloantigens.
Figures

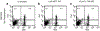



References
-
- Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors.] Lipid Res 2006;47(1):15–31. - PubMed
-
- Harousseau JL. Farnesyltransferase inhibitors in hematologic malignancies. Blood Rev 2007;21(4):173–82. - PubMed
-
- Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and - independent growth of human tumor cell lines. Cancer Res 1995;55(22):5302–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

