Dissociation of tau toxicity and phosphorylation: role of GSK-3beta, MARK and Cdk5 in a Drosophila model
- PMID: 18930955
- PMCID: PMC2644648
- DOI: 10.1093/hmg/ddn326
Dissociation of tau toxicity and phosphorylation: role of GSK-3beta, MARK and Cdk5 in a Drosophila model
Abstract
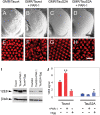
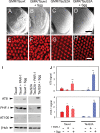
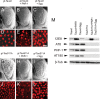
Hyperphosphorylation of tau at multiple sites has been implicated in the formation of neurofibrillary tangles in Alzheimer's disease; however, the relationship between toxicity and phosphorylation of tau has not been clearly elucidated. Putative tau kinases that play a role in such phosphorylation events include the proline-directed kinases glycogen synthase kinase-3beta (GSK-3beta) and cyclin-dependent kinase 5 (Cdk5), as well as nonproline-directed kinases such as microtubule affinity-regulating kinase (MARK)/PAR-1; however, whether the cascade of events linking tau phosphorylation and neurodegeneration involves sequential action of kinases as opposed to parallel pathways is still a matter of controversy. Here, we employed a well-characterized Drosophila model of tauopathy to investigate the interdependence of tau kinases in regulating the phosphorylation and toxicity of tau in vivo. We found that tau mutants resistant to phosphorylation by MARK/PAR-1 were indeed less toxic than wild-type tau; however, this was not due to their resistance to phosphorylation by GSK-3beta/Shaggy. On the contrary, a tau mutant resistant to phosphorylation by GSK-3beta/Shaggy retained substantial toxicity and was found to have increased affinity for microtubules compared with wild-type tau. The fly homologs of Cdk5/p35 did not have major effects on tau toxicity or phosphorylation in this model. These data suggest that, in addition to tau phosphorylation, microtubule binding plays a crucial role in the regulation of tau toxicity when misexpressed. These data have important implications for the understanding and interpretation of animal models of tauopathy.
Figures




References
-
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Watanabe A., Titani K., Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol. Aging. 1995;16:365–371. - PubMed
-
- Johnson G.V., Bailey C.D. Tau, where are we now? J. Alzheimers Dis. 2002;4:375–398. - PubMed
-
- Cho J.H., Johnson G.V. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 2003;278:187–193. - PubMed
-
- Augustinack J.C., Schneider A., Mandelkow E.M., Hyman B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. (Berl.) 2002;103:26–35. - PubMed
-
- Baum L., Hansen L., Masliah E., Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol. Chem. Neuropathol. 1996;29:253–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

