Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula
- PMID: 18931020
- PMCID: PMC2590744
- DOI: 10.1105/tpc.108.061739
Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula
Abstract
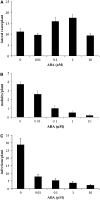
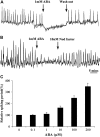
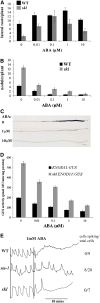
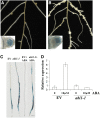
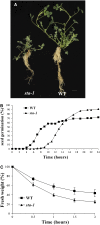
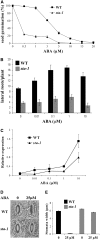
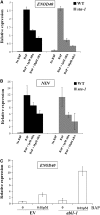
Nodulation is tightly regulated in legumes to ensure appropriate levels of nitrogen fixation without excessive depletion of carbon reserves. This balance is maintained by intimately linking nodulation and its regulation with plant hormones. It has previously been shown that ethylene and jasmonic acid (JA) are able to regulate nodulation and Nod factor signal transduction. Here, we characterize the nature of abscisic acid (ABA) regulation of nodulation. We show that application of ABA inhibits nodulation, bacterial infection, and nodulin gene expression in Medicago truncatula. ABA acts in a similar manner as JA and ethylene, regulating Nod factor signaling and affecting the nature of Nod factor-induced calcium spiking. However, this action is independent of the ethylene signal transduction pathway. We show that genetic inhibition of ABA signaling through the use of a dominant-negative allele of ABSCISIC ACID INSENSITIVE1 leads to a hypernodulation phenotype. In addition, we characterize a novel locus of M. truncatula, SENSITIVITY TO ABA, that dictates the sensitivity of the plant to ABA and, as such, impacts the regulation of nodulation. We show that ABA can suppress Nod factor signal transduction in the epidermis and can regulate cytokinin induction of the nodule primordium in the root cortex. Therefore, ABA is capable of coordinately regulating the diverse developmental pathways associated with nodule formation and can intimately dictate the nature of the plants' response to the symbiotic bacteria.
Figures


References
-
- Ane, J.M., et al. (2004). Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303 1364–1367. - PubMed
-
- Boisson-Dernier, A., Chabaud, M., Garcia, F., Becard, G., Rosenberg, C., and Barker, D.G. (2001). Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol. Plant Microbe Interact. 14 695–700. - PubMed
-
- Brady, S.M., Sarkar, S.F., Bonetta, D., and McCourt, P. (2003). The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 34 67–75. - PubMed
-
- Bright, L.J., Liang, Y., Mitchell, D.M., and Harris, J.M. (2005). The LATD gene of Medicago truncatula is required for both nodule and root development. Mol. Plant Microbe Interact. 18 521–532. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

