Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome
- PMID: 18931034
- PMCID: PMC2614532
- DOI: 10.1152/ajpheart.00185.2008
Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome
Abstract
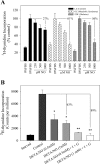
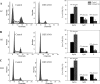
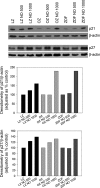
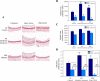
Type II diabetes mellitus (DM) and metabolic syndrome are associated with accelerated restenosis following vascular interventions due to neointimal hyperplasia. The efficacy of nitric oxide (NO)-based therapies is unknown in these environments. Therefore, the aim of this study is to examine the efficacy of NO in preventing neointimal hyperplasia in animal models of type II DM and metabolic syndrome and examine possible mechanisms for differences in outcomes. Aortic vascular smooth muscle cells (VSMC) were harvested from rodent models of type II DM (Zucker diabetic fatty), metabolic syndrome (obese Zucker), and their genetic control (lean Zucker). Interestingly, NO inhibited proliferation and induced G0/G1 cell cycle arrest to the greatest extent in VSMC from rodent models of metabolic syndrome and type II DM compared with controls. This heightened efficacy was associated with increased expression of cyclin-dependent kinase inhibitor p21, but not p27. Using the rat carotid artery injury model to assess the efficacy of NO in vivo, we found that the NO donor PROLI/NO inhibited neointimal hyperplasia to the greatest extent in type II DM rodents, followed by metabolic syndrome, then controls. Increased neointimal hyperplasia correlated with increased reactive oxygen species (ROS) production, as demonstrated by dihydroethidium staining, and NO inhibited this increase most in metabolic syndrome and DM. In conclusion, NO was surprisingly a more effective inhibitor of neointimal hyperplasia following arterial injury in type II DM and metabolic syndrome vs. control. This heightened efficacy may be secondary to greater inhibition of VSMC proliferation through cell cycle arrest and regulation of ROS expression, in addition to other possible unidentified mechanisms that deserve further exploration.
Figures



Similar articles
-
Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia.J Vasc Surg. 2008 Jan;47(1):173-82. doi: 10.1016/j.jvs.2007.09.005. J Vasc Surg. 2008. PMID: 18178471 Free PMC article.
-
Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10.Cell Biochem Biophys. 2011 Jun;60(1-2):89-97. doi: 10.1007/s12013-011-9179-3. Cell Biochem Biophys. 2011. PMID: 21448667 Free PMC article.
-
Insulin enhances the effect of nitric oxide at inhibiting neointimal hyperplasia in a rat model of type 1 diabetes.Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H772-9. doi: 10.1152/ajpheart.01234.2009. Epub 2010 Jun 18. Am J Physiol Heart Circ Physiol. 2010. PMID: 20562340 Free PMC article.
-
CNS-targeting pharmacological interventions for the metabolic syndrome.J Clin Invest. 2019 Aug 5;129(10):4058-4071. doi: 10.1172/JCI129195. eCollection 2019 Aug 5. J Clin Invest. 2019. PMID: 31380808 Free PMC article. Review.
-
Rodent models for metabolic syndrome research.J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. Epub 2010 Dec 30. J Biomed Biotechnol. 2011. PMID: 21253582 Free PMC article. Review.
Cited by
-
Effect of metabolic syndrome on the response to arterial injury.J Surg Res. 2014 Sep;191(1):33-41. doi: 10.1016/j.jss.2014.05.051. Epub 2014 May 27. J Surg Res. 2014. PMID: 24972735 Free PMC article.
-
Comparison of endothelial function of coronary artery bypass grafts in diabetic and nondiabetic patients: Which graft offers the best?Anatol J Cardiol. 2015 Aug;15(8):657-62. doi: 10.5152/akd.2014.5613. Anatol J Cardiol. 2015. PMID: 26301347 Free PMC article.
-
Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status.Free Radic Biol Med. 2011 May 1;50(9):1065-74. doi: 10.1016/j.freeradbiomed.2011.01.016. Epub 2011 Jan 21. Free Radic Biol Med. 2011. PMID: 21256959 Free PMC article.
-
Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury.J Vasc Surg. 2010 May;51(5):1248-59. doi: 10.1016/j.jvs.2009.12.028. Epub 2010 Mar 11. J Vasc Surg. 2010. PMID: 20223627 Free PMC article.
-
Role of metabolic environment on nitric oxide mediated inhibition of neointimal hyperplasia in type 1 and type 2 diabetes.Nitric Oxide. 2014 Jan 30;36:67-75. doi: 10.1016/j.niox.2013.12.005. Epub 2013 Dec 12. Nitric Oxide. 2014. PMID: 24333562 Free PMC article.
References
-
- Ahari A, Bergqvist D, Troeng T, Elfstrom J, Hedberg B, Ljungstrom KG, Norgren L, Ortenwall P. Diabetes mellitus as a risk factor for early outcome after carotid endarterectomy–a population-based study. Eur J Vasc Endovasc Eur J Vasc Endovasc 18: 122–126, 1999. - PubMed
-
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004. - PubMed
-
- Barbato JE, Zuckerbraun BS, Overhaus M, Raman KG, Tzeng E. Nitric oxide modulates vascular inflammation and intimal hyperplasia in insulin resistance and the metabolic syndrome. Am J Physiol Heart Circ Physiol 289: H228–H236, 2005. - PubMed
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287: 2570–2581, 2002. - PubMed
-
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63: 175–195, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

