Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways?
- PMID: 18931053
- PMCID: PMC2636953
- DOI: 10.1152/ajplung.90388.2008
Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways?
Abstract
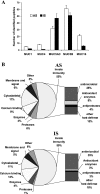
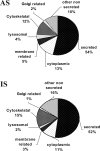
Human tracheobronchial epithelial cells grown in air-liquid interface culture have emerged as a powerful tool for the study of airway biology. In this study, we have investigated whether this culture system produces "mucus" with a protein composition similar to that of in vivo, induced airway secretions. Previous compositional studies of mucous secretions have greatly underrepresented the contribution of mucins, which are major structural components of normal mucus. To overcome this limitation, we have used a mass spectrometry-based approach centered on prior separation of the mucins from the majority of the other proteins. Using this approach, we have compared the protein composition of apical secretions (AS) from well-differentiated primary human tracheobronchial cells grown at air-liquid interface and human tracheobronchial normal induced sputum (IS). A total of 186 proteins were identified, 134 from AS and 136 from IS; 84 proteins were common to both secretions, with host defense proteins being predominant. The epithelial mucins MUC1, MUC4, and MUC16 and the gel-forming mucins MUC5B and MUC5AC were identified in both secretions. Refractometry showed that the gel-forming mucins were the major contributors by mass to both secretions. When the composition of the IS was corrected for proteins that were most likely derived from saliva, serum, and migratory cells, there was considerable similarity between the two secretions, in particular, in the category of host defense proteins, which includes the mucins. This shows that the primary cell culture system is an important model for study of aspects of innate defense of the upper airways related specifically to mucus consisting solely of airway cell products.
Figures


References
-
- Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol 25: 397–400, 2001. - PubMed
-
- Alexis NE, Soukup J, Nierkens S, Becker S. Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol 280: L369–L375, 2001. - PubMed
-
- Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 285: L730–L739, 2003. - PubMed
-
- Bacci E, Cianchetti S, Paggiaro PL, Carnevali S, Bancalari L, Dente FL, Di Franco A, Giannini D, Vagaggini B, Giuntini C. Comparison between hypertonic and isotonic saline-induced sputum in the evaluation of airway inflammation in subjects with moderate asthma. Clin Exp Allergy 26: 1395–1400, 1996. - PubMed
-
- Barnes FA, Bingle L, Bingle CD. Pulmonary genomics, proteomics, PLUNCs. Am J Respir Cell Mol Biol 38: 377–379, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

