A novel class of modular transporters for vitamins in prokaryotes
- PMID: 18931129
- PMCID: PMC2612444
- DOI: 10.1128/JB.01208-08
A novel class of modular transporters for vitamins in prokaryotes
Abstract
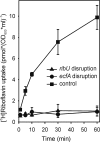
The specific and tightly controlled transport of numerous nutrients and metabolites across cellular membranes is crucial to all forms of life. However, many of the transporter proteins involved have yet to be identified, including the vitamin transporters in various human pathogens, whose growth depends strictly on vitamin uptake. Comparative analysis of the ever-growing collection of microbial genomes coupled with experimental validation enables the discovery of such transporters. Here, we used this approach to discover an abundant class of vitamin transporters in prokaryotes with an unprecedented architecture. These transporters have energy-coupling modules comprised of a conserved transmembrane protein and two nucleotide binding proteins similar to those of ATP binding cassette (ABC) transporters, but unlike ABC transporters, they use small integral membrane proteins to capture specific substrates. We identified 21 families of these substrate capture proteins, each with a different specificity predicted by genome context analyses. Roughly half of the substrate capture proteins (335 cases) have a dedicated energizing module, but in 459 cases distributed among almost 100 gram-positive bacteria, including numerous human pathogens, different and unrelated substrate capture proteins share the same energy-coupling module. The shared use of energy-coupling modules was experimentally confirmed for folate, thiamine, and riboflavin transporters. We propose the name energy-coupling factor transporters for the new class of membrane transporters.
Figures




References
-
- Davidson, A. L., and J. Chen. 2004. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73241-268. - PubMed
-
- Duurkens, R. H., M. B. Tol, E. R. Geertsma, H. P. Permentier, and D. J. Slotboom. 2007. Flavin binding to the high affinity riboflavin transporter RibU. J. Biol. Chem. 28210380-10386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

