Spontaneous purinergic neurotransmission in the mouse urinary bladder
- PMID: 18936079
- PMCID: PMC2655397
- DOI: 10.1113/jphysiol.2008.162040
Spontaneous purinergic neurotransmission in the mouse urinary bladder
Abstract
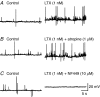
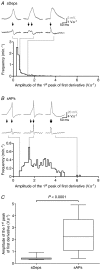
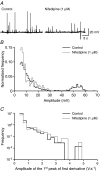
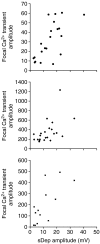
Spontaneous purinergic neurotransmission was characterized in the mouse urinary bladder, a model for the pathological or ageing human bladder. Intracellular electrophysiological recording from smooth muscle cells of the detrusor muscle revealed spontaneous depolarizations, distinguishable from spontaneous action potentials (sAPs) by their amplitude (< 40 mV) and insensitivity to the L-type Ca(2+) channel blocker nifedipine (1 microm) (100 +/- 29%). Spontaneous depolarizations were abolished by the P2X(1) receptor antagonist NF449 (10 microm) (frequency 8.5 +/- 8.5% of controls), insensitive to the muscarinic acetylcholine receptor antagonist atropine (1 microm) (103.4 +/- 3.0%), and became more frequent in latrotoxin (LTX; 1 nm) (438 +/- 95%), suggesting that they are spontaneous excitatory junction potentials (sEJPs). Such sEJPs were correlated, in amplitude and timing, with focal Ca(2+) transients in smooth muscle cells (measured using confocal microscopy), suggesting a common origin: ATP binding to P2X(1) receptors. sAPs were abolished by NF449, insensitive to atropine (126 +/- 39%) and increased in frequency by LTX (930 +/- 450%) suggesting a neurogenic, purinergic origin, in common with sEJPs. By comparing the kinetics of sAPs and sEJPs, we demonstrated that sAPs occur when sufficient cation influx through P2X(1) receptors triggers L-type Ca(2+) channels; the first peak of the differentiated rising phase of depolarizations - attributed to the influx of cations through the P2X(1) receptor - is of larger amplitude for sAPs (2248 mV s(-1)) than sEJPs (439 mV s(-1)). Surprisingly, sAPs in the mouse urinary bladder, unlike those from other species, are triggered by stochastic ATP release from parasympathetic nerve terminals rather than being myogenic.
Figures


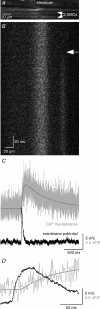
 , where ΔF is the change in fluorescence, Fo the resting fluorescence, and ‘area’ is the area over which the fluorescent signal is measured (in pixels) divided by 32; this is hence an arbitrary unit.
, where ΔF is the change in fluorescence, Fo the resting fluorescence, and ‘area’ is the area over which the fluorescent signal is measured (in pixels) divided by 32; this is hence an arbitrary unit.References
-
- Blair DH, Lin YQ, Bennett MR. Differential sensitivity to calcium and osmotic pressure of fast and slow ATP currents at sympathetic varicosities in mouse vas deferens. Auton Neurosci. 2003;105:45–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

