Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat
- PMID: 18936104
- PMCID: PMC4236191
- DOI: 10.1136/gut.2008.157594
Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat
Abstract
Background: Increasing evidence suggests that chronic stress plays an important role in the pathophysiology of several functional gastrointestinal disorders. We investigated whether cannabinoid receptor 1 (CB1) and vanilloid receptor 1 (TRPV1; transient receptor potential vanilloid 1) are involved in stress-induced visceral hyperalgesia.
Methods: Male rats were exposed to 1 h water avoidance (WA) stress daily for 10 consecutive days. The visceromotor response (VMR) to colorectal distension (CRD) was measured. Immunofluorescence and western blot analysis were used to assess the expression of CB1 and TRPV1 receptors in dorsal root ganglion (DRG) neurons.
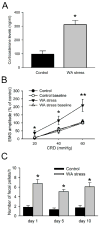
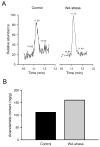
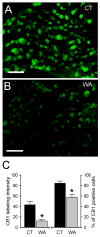
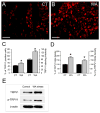
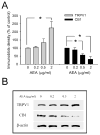
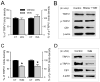
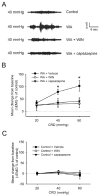
Results: WA stressed rats demonstrated a significant increase in the serum corticosterone levels and faecal pellet output compared to controls supporting stimulation of the hypothalamic-pituitary-adrenal (HPA) axis. The VMR increased significantly at pressures of 40 and 60 mm Hg in WA stress rats compared with controls, respectively, and was associated with hyperalgesia. The endogenous CB1 agonist anandamide was increased significantly in DRGs from stressed rats. Immunofluorescence and western blot analysis showed a significant decrease in CB1 and a reciprocal increase in TRPV1 expression and phosphorylation in DRG neurons from stressed rats. These reciprocal changes in CB1 and TRPV1 were reproduced by treatment of control DRGs with anandamide in vitro. In contrast, treatment of control DRGs in vitro with the CB1 receptor agonist WIN 55,212-2 decreased the levels of TRPV1 and TRPV1 phosphorylation. Treatment of WA stress rats in situ with WIN 55,212-2 or the TRPV1 antagonist capsazepine prevented the development of visceral hyperalgesia and blocked the upregulation of TRPV1.
Conclusions: These results suggest that the endocannabinoid (CB1) and TRP (TRPV1) pathways may play a potentially important role in stress-induced visceral hyperalgesia.
Conflict of interest statement
Figures

Similar articles
-
Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat.Gastroenterology. 2011 Feb;140(2):627-637.e4. doi: 10.1053/j.gastro.2010.11.003. Epub 2010 Nov 9. Gastroenterology. 2011. PMID: 21070780 Free PMC article.
-
Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways.Exp Neurol. 2015 Nov;273:301-11. doi: 10.1016/j.expneurol.2015.09.013. Epub 2015 Sep 25. Exp Neurol. 2015. PMID: 26408049 Free PMC article.
-
Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system.Gastroenterology. 2015 Jan;148(1):148-157.e7. doi: 10.1053/j.gastro.2014.09.032. Epub 2014 Sep 28. Gastroenterology. 2015. PMID: 25263804 Free PMC article.
-
Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity.Mol Neurobiol. 2007 Jun;35(3):245-54. doi: 10.1007/s12035-007-0030-1. Mol Neurobiol. 2007. PMID: 17917113 Review.
-
Interplay between endocannabinoid and endovanilloid mechanisms in fear conditioning.Acta Neuropsychiatr. 2024 Oct;36(5):255-264. doi: 10.1017/neu.2023.54. Epub 2023 Nov 20. Acta Neuropsychiatr. 2024. PMID: 37982167 Review.
Cited by
-
Opposing Roles of Estradiol and Testosterone on Stress-Induced Visceral Hypersensitivity in Rats.J Pain. 2018 Jul;19(7):764-776. doi: 10.1016/j.jpain.2018.02.007. Epub 2018 Mar 2. J Pain. 2018. PMID: 29496640 Free PMC article.
-
Maladaptive changes in the homeostasis of AEA-TRPV1/CB1R induces pain-related hyperactivity of nociceptors after spinal cord injury.Cell Biosci. 2025 Jan 9;15(1):2. doi: 10.1186/s13578-025-01345-6. Cell Biosci. 2025. PMID: 39789637 Free PMC article.
-
Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain.Pharmaceuticals (Basel). 2010 Aug 26;3(9):2768-2798. doi: 10.3390/ph3092768. Pharmaceuticals (Basel). 2010. PMID: 27713376 Free PMC article. Review.
-
Increased expression of cannabinoid CB₁ receptors in Achilles tendinosis.PLoS One. 2011;6(9):e24731. doi: 10.1371/journal.pone.0024731. Epub 2011 Sep 8. PLoS One. 2011. PMID: 21931835 Free PMC article.
-
Colitis-Induced Microbial Perturbation Promotes Postinflammatory Visceral Hypersensitivity.Cell Mol Gastroenterol Hepatol. 2020;10(2):225-244. doi: 10.1016/j.jcmgh.2020.04.003. Epub 2020 Apr 11. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32289500 Free PMC article.
References
-
- Mayer EA, Naliboff BD, Chang L, Coutinho SVV. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. - PubMed
-
- Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. - PubMed
-
- Carrier EJ, Patel S, Hillard CJ. Endocannabinoids in neuroimmunology and stress. Curr Drug Targets CNS Neurol Disord. 2005;4:657–665. - PubMed
-
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
