Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina
- PMID: 18936150
- PMCID: PMC2678967
- DOI: 10.1167/iovs.08-2499
Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina
Abstract
Purpose: To determine whether intraocular pressure (IOP) elevation alters OPA1 expression and triggers OPA1 release, as well as whether the uncompetitive N-methyl-d-aspartate (NMDA) glutamate receptor antagonist memantine blocks OPA1 release and subsequent apoptotic cell death in glaucomatous DBA/2J mouse retina.
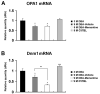
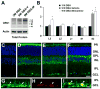
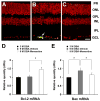
Methods: Preglaucomatous DBA/2J mice received memantine (5 mg/kg, intraperitoneal injection, twice daily for 3 months) and IOP in the eyes was measured monthly. RGC loss was counted after FluoroGold labeling. OPA1, Dnm1, Bcl-2, and Bax mRNA were measured by qPCR. OPA1 protein was assessed by immunohistochemistry and Western blot. Apoptotic cell death was assessed by TUNEL staining.
Results: Memantine treatment significantly increased RGC survival in glaucomatous DBA/2J mice and increased the 75-kDa OPA1 isoform, but did not alter the 80- and 90-kDa isoforms. The isoforms of OPA1 were significantly increased in the cytosol of the vehicle-treated glaucomatous retinas but were significantly decreased in memantine-treated glaucomatous retinas. OPA1 immunoreactivity was decreased in the photoreceptors of both vehicle- and memantine-treated glaucomatous retinas, but was increased in the outer plexiform layer of only the memantine-treated glaucomatous retinas. Memantine blocked apoptotic cell death in the GCL, increased Bcl-2 gene expression, and decreased Bax gene expression.
Conclusions: OPA1 release from mitochondria in glaucomatous mouse retina is inhibited by blockade of glutamate receptor activation. Because this OPA1 effect was accompanied by increased Bcl-2 expression, decreased Bax expression, and apoptosis blockade, glutamate receptor activation in the glaucomatous retina may involve a distinct mitochondria-mediated cell death pathway.
Figures



References
-
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. - PubMed
-
- Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48(Suppl 1):S38–46. - PubMed
-
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38(3):357–366. - PubMed
-
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. - PubMed
-
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2000;4(2):149–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

