Fragile X mental retardation protein FMRP binds mRNAs in the nucleus
- PMID: 18936162
- PMCID: PMC2612477
- DOI: 10.1128/MCB.01377-08
Fragile X mental retardation protein FMRP binds mRNAs in the nucleus
Abstract
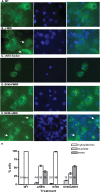
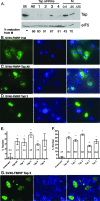
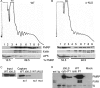
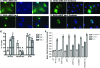
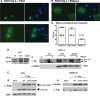
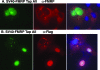
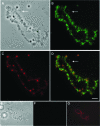
The fragile X mental retardation protein FMRP is an RNA binding protein that associates with a large collection of mRNAs. Since FMRP was previously shown to be a nucleocytoplasmic shuttling protein, we examined the hypothesis that FMRP binds its cargo mRNAs in the nucleus. The enhanced green fluorescent protein-tagged FMRP construct (EGFP-FMRP) expressed in Cos-7 cells was efficiently exported from the nucleus in the absence of its nuclear export sequence and in the presence of a strong nuclear localization sequence (the simian virus 40 [SV40] NLS), suggesting an efficient mechanism for nuclear export. We hypothesized that nuclear FMRP exits the nucleus through its bound mRNAs. Using silencing RNAs to the bulk mRNA exporter Tap/NXF1, we observed a significantly increased number of cells containing EGFP-FMRP in the nucleus, which was further augmented by removal of FMRP's nuclear export sequence. Nuclear-retained SV40-FMRP could be released upon treatment with RNase. Further, Tap/NXF1 coimmunoprecipitated with EGFP-FMRP in an RNA-dependent manner and contained the FMR1 mRNA. To determine whether FMRP binds pre-mRNAs cotranscriptionally, we expressed hemagglutinin-SV40 FMRP in amphibian oocytes and found it, as well as endogenous Xenopus FMRP, on the active transcription units of lampbrush chromosomes. Collectively, our data provide the first lines of evidence that FMRP binds mRNA in the nucleus.
Figures
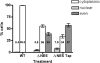

Similar articles
-
The fragile X mental retardation protein interacts with a distinct mRNA nuclear export factor NXF2.RNA. 2006 Aug;12(8):1446-9. doi: 10.1261/rna.94306. Epub 2006 Jun 21. RNA. 2006. PMID: 16790844 Free PMC article.
-
Expression of fragile X mental retardation-1 gene with nuclear export signal mutation changes the expression profiling of mouse cerebella immortal neuronal cell.Proteomics. 2005 Oct;5(15):3979-90. doi: 10.1002/pmic.200401252. Proteomics. 2005. PMID: 16130171
-
The RNA-binding protein FMRP facilitates the nuclear export of N6-methyladenosine-containing mRNAs.J Biol Chem. 2019 Dec 27;294(52):19889-19895. doi: 10.1074/jbc.AC119.010078. Epub 2019 Nov 21. J Biol Chem. 2019. PMID: 31753916 Free PMC article.
-
Fragile X mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability.Mol Cell Neurosci. 2010 Jan;43(1):43-50. doi: 10.1016/j.mcn.2009.09.013. Epub 2009 Oct 22. Mol Cell Neurosci. 2010. PMID: 19837168 Review.
-
Identification of messenger RNAs and microRNAs associated with fragile X mental retardation protein.Methods Mol Biol. 2006;342:267-76. doi: 10.1385/1-59745-123-1:267. Methods Mol Biol. 2006. PMID: 16957381 Review.
Cited by
-
Replication Stress Induces Global Chromosome Breakage in the Fragile X Genome.Cell Rep. 2020 Sep 22;32(12):108179. doi: 10.1016/j.celrep.2020.108179. Cell Rep. 2020. PMID: 32966779 Free PMC article.
-
A new regulatory function of the region proximal to the RGG box in the fragile X mental retardation protein.J Cell Sci. 2011 Sep 15;124(Pt 18):3060-5. doi: 10.1242/jcs.086751. Epub 2011 Aug 24. J Cell Sci. 2011. PMID: 21868366 Free PMC article.
-
Structural studies of the tandem Tudor domains of fragile X mental retardation related proteins FXR1 and FXR2.PLoS One. 2010 Nov 2;5(11):e13559. doi: 10.1371/journal.pone.0013559. PLoS One. 2010. PMID: 21072162 Free PMC article.
-
Astrocytes in fragile X syndrome.Front Cell Neurosci. 2024 Jan 8;17:1322541. doi: 10.3389/fncel.2023.1322541. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38259499 Free PMC article. Review.
-
Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males.Am J Med Genet A. 2010 Oct;152A(10):2512-20. doi: 10.1002/ajmg.a.33626. Am J Med Genet A. 2010. PMID: 20799337 Free PMC article.
References
-
- Adinolfi, S., A. Ramos, S. R. Martin, F. Dal Piaz, P. Pucci, B. Bardoni, J. L. Mandel, and A. Pastore. 2003. The N-terminus of the fragile X mental retardation protein contains a novel domain involved in dimerization and RNA binding. Biochemistry 4210437-10444. - PubMed
-
- Arttamangkul, S., V. Alvarez-Maubecin, G. Thomas, J. T. Williams, and D. K. Grandy. 2000. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol. Pharmacol. 581570-1580. - PubMed
-
- Ashley, C. T., J. S. Sutcliffe, C. B. Kunst, H. A. Leiner, E. E. Eichler, D. L. Nelson, and S. T. Warren. 1993. Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat. Genet. 4244-251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
