Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning
- PMID: 18936328
- PMCID: PMC2676165
- DOI: 10.1161/CIRCULATIONAHA.108.788331
Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning
Abstract
Background: Caveolae, lipid-rich microdomains of the sarcolemma, localize and enrich cardiac-protective signaling molecules. Caveolin-3 (Cav-3), the dominant isoform in cardiac myocytes, is a determinant of caveolar formation. We hypothesized that cardiac myocyte-specific overexpression of Cav-3 would enhance the formation of caveolae and augment cardiac protection in vivo.
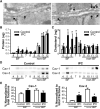
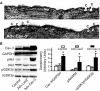
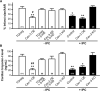
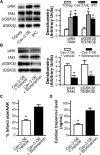
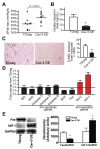
Methods and results: Ischemic preconditioning in vivo increased the formation of caveolae. Adenovirus for Cav-3 increased caveolar formation and phosphorylation of survival kinases in cardiac myocytes. A transgenic mouse with cardiac myocyte-specific overexpression of Cav-3 (Cav-3 OE) showed enhanced formation of caveolae on the sarcolemma. Cav-3 OE mice subjected to ischemia/reperfusion injury had a significantly reduced infarct size relative to transgene-negative mice. Endogenous cardiac protection in Cav-3 OE mice was similar to wild-type mice undergoing ischemic preconditioning; no increased protection was observed in preconditioned Cav-3 OE mice. Cav-3 knockout mice did not show endogenous protection and showed no protection in response to ischemic preconditioning. Cav-3 OE mouse hearts had increased basal Akt and glycogen synthase kinase-3beta phosphorylation comparable to wild-type mice exposed to ischemic preconditioning. Wortmannin, a phosphoinositide 3-kinase inhibitor, attenuated basal phosphorylation of Akt and glycogen synthase kinase-3beta and blocked cardiac protection in Cav-3 OE mice. Cav-3 OE mice had improved functional recovery and reduced apoptosis at 24 hours of reperfusion.
Conclusions: Expression of caveolin-3 is both necessary and sufficient for cardiac protection, a conclusion that unites long-standing ultrastructural and molecular observations in the ischemic heart. The present results indicate that increased expression of caveolins, apparently via actions that depend on phosphoinositide 3-kinase, has the potential to protect hearts exposed to ischemia/reperfusion injury.
Figures



References
-
- Noble R. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am J Physiol. 1943;38:346–351.
-
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. - PubMed
-
- Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. - PubMed
-
- Steinberg SF, Brunton LL. Compartmentation of g protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

