Development of eye-movement control
- PMID: 18938009
- PMCID: PMC2731686
- DOI: 10.1016/j.bandc.2008.08.019
Development of eye-movement control
Abstract
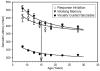
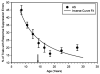
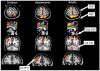
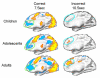
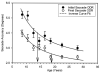
Cognitive control of behavior continues to improve through adolescence in parallel with important brain maturational processes including synaptic pruning and myelination, which allow for efficient neuronal computations and the functional integration of widely distributed circuitries supporting top-down control of behavior. This is also a time when psychiatric disorders, such as schizophrenia and mood disorders, emerge reflecting a particularly vulnerability to impairments in development during adolescence. Oculomotor studies provide a unique neuroscientific approach to make precise associations between cognitive control and brain circuitry during development that can inform us of impaired systems in psychopathology. In this review, we first describe the development of pursuit, fixation, and visually-guided saccadic eye movements, which collectively indicate early maturation of basic sensorimotor processes supporting reflexive, exogenously-driven eye movements. We then describe the literature on the development of the cognitive control of eye movements as reflected in the ability to inhibit a prepotent eye movement in the antisaccade task, as well as making an eye movement guided by on-line spatial information in working memory in the oculomotor delayed response task. Results indicate that the ability to make eye movements in a voluntary fashion driven by endogenous plans shows a protracted development into adolescence. Characterizing the transition through adolescence to adult-level cognitive control of behavior can inform models aimed at understanding the neurodevelopmental basis of psychiatric disorders.
Figures



References
-
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology. 2000;84:2166–2170. - PubMed
-
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. Journal of Neurophysiology. 2004;91:1672–1689. - PubMed
-
- Andersen SL. Stimulants and the developing brain. Trends in Pharmacological Sciences. 2005;26:237–243. - PubMed
-
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. - PubMed
-
- Aring E, Grönlund MA, Hellström A, Ygge J. Visual fixation development in children. Graefes Archives of Clinical and Experimental Opthalmology. 2007;245:1659–1665. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

