A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance
- PMID: 18938181
- PMCID: PMC2657297
- DOI: 10.1016/j.jtbi.2008.09.012
A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance
Abstract
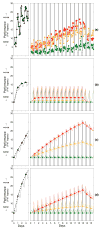
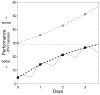
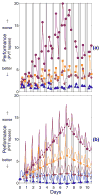
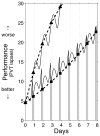
The two-process model of sleep regulation makes accurate predictions of sleep timing and duration for a variety of experimental sleep deprivation and nap sleep scenarios. Upon extending its application to waking neurobehavioral performance, however, the model fails to predict the effects of chronic sleep restriction. Here we show that the two-process model belongs to a broader class of models formulated in terms of coupled non-homogeneous first-order ordinary differential equations, which have a dynamic repertoire capturing waking neurobehavioral functions across a wide range of wake/sleep schedules. We examine a specific case of this new model class, and demonstrate the existence of a bifurcation: for daily amounts of wakefulness less than a critical threshold, neurobehavioral performance is predicted to converge to an asymptotically stable state of equilibrium; whereas for daily wakefulness extended beyond the critical threshold, neurobehavioral performance is predicted to diverge from an unstable state of equilibrium. Comparison of model simulations to laboratory observations of lapses of attention on a psychomotor vigilance test (PVT), in experiments on the effects of chronic sleep restriction and acute total sleep deprivation, suggests that this bifurcation is an essential feature of performance impairment due to sleep loss. We present three new predictions that may be experimentally verified to validate the model. These predictions, if confirmed, challenge conventional notions about the effects of sleep and sleep loss on neurobehavioral performance. The new model class implicates a biological system analogous to two connected compartments containing interacting compounds with time-varying concentrations as being a key mechanism for the regulation of psychomotor vigilance as a function of sleep loss. We suggest that the adenosinergic neuromodulator/receptor system may provide the underlying neurobiology.
Figures


References
-
- Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75:A37–A43. - PubMed
-
- Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern. 1994;71:115–121. - PubMed
-
- Åkerstedt T, Folkard S. The three-process model of alertness and its extension to performance, sleep latency, and sleep length. Chronobiol Int. 1997;14:115–123. - PubMed
-
- Avinash D, Crudele CP, Amin DD, Robinson BM, Dinges DF, Van Dongen HPA. Parameter estimation for a biomathematical model of psychomotor vigilance performance under laboratory conditions of chronic sleep restriction. In: Ruigt GSF, Van Bemmel AL, DeBoer T, Hofman WF, Van Luijtelaar G, editors. Sleep-Wake Research in the Netherlands. Vol. 16. Dutch Society for Sleep-Wake Research; The Netherlands: 2005. pp. 39–42.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

