Rapid enantiomeric excess and concentration determination using simple racemic metal complexes
- PMID: 18939802
- PMCID: PMC2749327
- DOI: 10.1021/ol802085j
Rapid enantiomeric excess and concentration determination using simple racemic metal complexes
Abstract
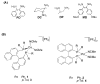
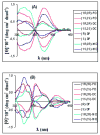
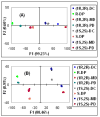
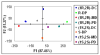
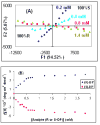
Racemic-metal complexes were used to determine identity, enantiomeric excess, and concentration of chiral diamines using metal-to-ligand charge transfer bands in circular dichroism spectroscopy. It takes under just 2 min per sample to determine [G]t and %R with tolerable errors (19% and 4%, respectively). The simplicity of the achiral receptors employed confers to this technique great potential for high-throughput screening.
Figures
References
-
- Welch CJ, Szczerba T, Perrin SR. J Chromatogr A. 1997;758:93–98.
- Welch CJ, Grau B, Moore J, Mathre DJ. J Org Chem. 2001;66:6836–6837. - PubMed
- Welch CJ, Fleitz F, Antia F, Yehl P, Waters R, Ikemoto N, Armstrong IJD, Mathre DJ. Org Process Res Dev. 2004;8:186–191.
- Sigman MS, Jacobsen EN. J Am Chem Soc. 1998;120:4901–4902.
- Wolf C, Hawes PA. J Org Chem. 2002;67:2727–2729. - PubMed
-
-
For recently publications see: Bailey DM, Hennig A, Uzunova VD, Nau WM. Chem Eur J. 2008;14:6069–6077.Truppo MD, Escalettes F, Turner NJ. Angew Chem Int Ed. 2008;47:2639–2641.Chen Z-H, He Y-B, Hu C-G, Huang X-H, Hu L. Aust J Chem. 2008;61:310–315.Ingle JR, Busch KW, Kenneth W, Busch MA. Talanta. 2008;75:572–584.Tanaka H, Matilde S. Chirality. 2008;20:307–312.Yashima E, Katsuhiro M. Macromolecules. 2008;41:3–12.Young BL, Cooks RG. Int J Mass Spectrom. 2007;267:199–204.Li Z-B, Lin J, Sabat M, Hyacinth M, Pu L. J Org Chem. 2007;72:4905–4916.
-
-
- Lin J, Zhang HC, Pu L. Org Lett. 2002;4:3297–3300. - PubMed
- Lee SJ, Lin W. J Am Chem Soc. 2002;124:4554–4555. - PubMed
- Ahn KH, Ku H-Y, Kim Y, Kim S-G, Kim YK, Son HS, Ku JK. Org Lett. 2003;5:1419–1422. - PubMed
- Corradini R, Paganuzzi C, Marchelli R, Pagliari S, Sforza S, Dossena A, Galaverna G, Duchateau J Mater Chem. 2005;15:2741–2746. - PubMed
-
- Zhu L, Anslyn EV. J Am Chem Soc. 2004;126:3676–3677. - PubMed
- Folmer-Andersen JF, Lynch VM, Anslyn EV. J Am Chem Soc. 2005;127:7986–7987. - PubMed
- Zhu L, Zhong Z, Anslyn EV. J Am Chem Soc. 2005;127:4260–4269. - PubMed
- Zhu L, Shabbir SH, Anslyn EV. Chem Eur J. 2006;13:99–104. - PubMed
- Folmer-Andersen JF, Kitamura M, Anslyn EV. J Am Chem Soc. 2006;128:5652–5653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

