Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy
- PMID: 18939816
- PMCID: PMC2661425
- DOI: 10.1021/jm800719t
Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy
Abstract
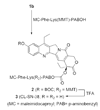
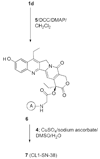
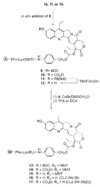
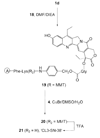
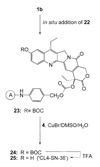
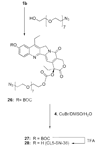
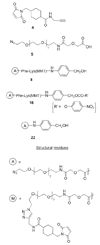
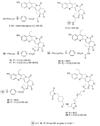
CPT-11 is a clinically used cancer drug, and it is a prodrug of the potent topoisomerase I inhibitor, SN-38 (7-ethyl-10-hydroxycamptothecin). To bypass the need for the in vivo conversion of CPT-11 and increase the therapeutic index, bifunctional derivatives of SN-38 were prepared for use in antibody-based targeted therapy of cancer. The general synthetic scheme incorporated an acetylene-azide click cycloaddition step in the design, a short polyethylene glycol spacer for aqueous solubility, and a maleimide group for conjugation. Conjugates of a humanized anti-CEACAM5 monoclonal antibody, hMN-14, prepared using these SN-38 derivatives were evaluated in vitro for stability in buffer and human serum and for antigen-binding and cytotoxicity in a human colon adenocarcinoma cell line. Conjugates of hMN-14 and SN-38 derivatives 16 and 17 were found promising for further development.
Figures



References
-
- Potmesil M. Camptothecins: from bench research to hospital wards. Cancer Res. 1994;54:1431–1439. - PubMed
-
- Sawada S, Okajima S, Aiyama R, Nokata K, Furuta T, Yokokura T, Sugino E, Yamaguchi K, Miyasaka T. Synthesis and antitumor activity of 20(S)-camptothecin derivatives: carbamate-linked, water-soluble derivatives of 7-ethyl-10-hydroxycamptothecin. Chem. Pharm. Bull. (Tokyo) 1991;39:1446–1450. - PubMed
-
- Rivory LP, Bowles MR, Robert J, Pond SM. Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochem. Pharmacol. 1996;52:1103–1111. - PubMed
-
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. - PubMed
-
- Rougier P, Bugat R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Seminars Oncol. (suppl. 3) 1996;23:34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

