Regulation of gene expression of hepatic drug metabolizing enzymes and transporters by the Toll-like receptor 2 ligand, lipoteichoic acid
- PMID: 18940178
- PMCID: PMC2635100
- DOI: 10.1016/j.abb.2008.10.003
Regulation of gene expression of hepatic drug metabolizing enzymes and transporters by the Toll-like receptor 2 ligand, lipoteichoic acid
Abstract
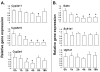
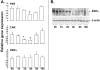
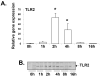
Expression of hepatic drug metabolizing enzymes (DMEs) is altered in infection and inflammation. However, the role of Gram+ve bacterial components and their receptor, Toll-like receptor (TLR) 2 in regulation of hepatic DMEs is unknown. Gene expression of DMEs is regulated by members of the nuclear receptor superfamily (PXR, CAR and RXRalpha). The TLR2 ligand, lipoteichoic acid (LTA) reduced RNA levels of CAR and its target genes, Cyp2b10, Cyp2a4 and Sultn in mouse liver ( approximately 60-80% reduction). Hepatic genes regulated by PXR and CAR, Cyp3a11 and Mrp2 were moderately reduced by LTA, along with approximately 50% reduction of PXR RNA and nuclear protein levels of RXRalpha. The effects of LTA were significantly attenuated by pre-treatment with the Kupffer cell inhibitor, gadolinium chloride, indicating that Kupffer cells contribute to LTA-mediated down-regulation of hepatic genes. These results indicate that treatment with Gram+ve bacterial components preferentially down-regulate CAR and its target genes in the liver.
Figures




Similar articles
-
Differential role of Toll-interleukin 1 receptor domain-containing adaptor protein in Toll-like receptor 2-mediated regulation of gene expression of hepatic cytokines and drug-metabolizing enzymes.Drug Metab Dispos. 2011 May;39(5):874-81. doi: 10.1124/dmd.110.037382. Epub 2011 Feb 8. Drug Metab Dispos. 2011. PMID: 21303924 Free PMC article.
-
Role of constitutive androstane receptor in Toll-like receptor-mediated regulation of gene expression of hepatic drug-metabolizing enzymes and transporters.Drug Metab Dispos. 2014 Jan;42(1):172-81. doi: 10.1124/dmd.113.053850. Epub 2013 Nov 5. Drug Metab Dispos. 2014. PMID: 24194512 Free PMC article.
-
Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters.Life Sci. 2011 Jul 4;89(1-2):57-64. doi: 10.1016/j.lfs.2011.05.005. Epub 2011 May 18. Life Sci. 2011. PMID: 21620874 Free PMC article.
-
Induction of phase I, II and III drug metabolism/transport by xenobiotics.Arch Pharm Res. 2005 Mar;28(3):249-68. doi: 10.1007/BF02977789. Arch Pharm Res. 2005. PMID: 15832810 Review.
-
Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR.Adv Drug Deliv Rev. 2010 Oct 30;62(13):1238-49. doi: 10.1016/j.addr.2010.08.006. Epub 2010 Aug 17. Adv Drug Deliv Rev. 2010. PMID: 20727377 Free PMC article. Review.
Cited by
-
Differential role of Toll-interleukin 1 receptor domain-containing adaptor protein in Toll-like receptor 2-mediated regulation of gene expression of hepatic cytokines and drug-metabolizing enzymes.Drug Metab Dispos. 2011 May;39(5):874-81. doi: 10.1124/dmd.110.037382. Epub 2011 Feb 8. Drug Metab Dispos. 2011. PMID: 21303924 Free PMC article.
-
CYP3A-dependent drug metabolism is reduced in bacterial inflammation in mice.Br J Pharmacol. 2012 Aug;166(7):2176-87. doi: 10.1111/j.1476-5381.2012.01933.x. Br J Pharmacol. 2012. PMID: 22394353 Free PMC article.
-
Role of Toll-like receptor 4 in drug-drug interaction between paclitaxel and irinotecan in vitro.Toxicol In Vitro. 2017 Jun;41:75-82. doi: 10.1016/j.tiv.2017.02.019. Epub 2017 Feb 27. Toxicol In Vitro. 2017. PMID: 28242239 Free PMC article.
-
Role of Adaptor Protein Toll-Like Interleukin Domain Containing Adaptor Inducing Interferon β in Toll-Like Receptor 3- and 4-Mediated Regulation of Hepatic Drug Metabolizing Enzyme and Transporter Genes.Drug Metab Dispos. 2016 Jan;44(1):61-7. doi: 10.1124/dmd.115.066761. Epub 2015 Oct 15. Drug Metab Dispos. 2016. PMID: 26470915 Free PMC article.
-
Drug disposition in pathophysiological conditions.Curr Drug Metab. 2012 Nov;13(9):1327-44. doi: 10.2174/138920012803341302. Curr Drug Metab. 2012. PMID: 22746301 Free PMC article. Review.
References
-
- Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–49. - PubMed
-
- Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol. 2005;1:629–40. - PubMed
-
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. - PubMed
-
- Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

