Targeting Dyrk1A with AAVshRNA attenuates motor alterations in TgDyrk1A, a mouse model of Down syndrome
- PMID: 18940310
- PMCID: PMC2561933
- DOI: 10.1016/j.ajhg.2008.09.010
Targeting Dyrk1A with AAVshRNA attenuates motor alterations in TgDyrk1A, a mouse model of Down syndrome
Abstract
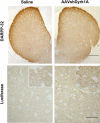
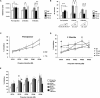
Genetic-dissection studies carried out with Down syndrome (DS) murine models point to the critical contribution of Dyrk1A overexpression to the motor abnormalities and cognitive deficits displayed in DS individuals. In the present study we have used a murine model overexpressing Dyrk1A (TgDyrk1A mice) to evaluate whether functional CNS defects could be corrected with an inhibitory RNA against Dyrk1A, delivered by bilateral intrastriatal injections of adeno-associated virus type 2 (AAVshDyrk1A). We report that AAVshDyrk1A efficiently transduced HEK293 cells and primary neuronal cultures, triggering the specific inhibition of Dyrk1A expression. Injecting the vector into the striata of TgDyrk1A mice resulted in a restricted, long-term transduction of the striatum. This gene therapy was found to be devoid of toxicity and succeeded in normalizing Dyrk1A protein levels in TgDyrk1A mice. Importantly, the behavioral studies of the adult TgDyrk1A mice treated showed a reversal of corticostriatal-dependent phenotypes, as revealed by the attenuation of their hyperactive behavior, the restoration of motor-coordination defects, and an improvement in sensorimotor gating. Taken together, the data demonstrate that normalizing Dyrk1A gene expression in the striatum of adult TgDyrk1A mice, by means of AAVshRNA, clearly reverses motor impairment. Furthermore, these results identify Dyrk1A as a potential target for therapy in DS.
Figures



References
-
- Hattori M., Fujiyama A., Taylor T.D., Watanabe H., Yada T., Park H.S., Toyoda A., Ishii K., Totoki Y., Choi D.K. The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. - PubMed
-
- Antonarakis S.E., Epstein C.J. The challenge of Down syndrome. Trends Mol. Med. 2006;12:473–479. - PubMed
-
- Guimera J., Casas C., Estivill X., Pritchard M. Human minibrain homologue (MNBH/DYRK1): Characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics. 1999;57:407–418. - PubMed
-
- Becker W., Weber Y., Wetzel K., Eirmbter K., Tejedor F.J., Joost H.G. Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J. Biol. Chem. 1998;273:25893–25902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

