Context-dependent changes in functional circuitry in visual area MT
- PMID: 18940596
- PMCID: PMC2652654
- DOI: 10.1016/j.neuron.2008.08.007
Context-dependent changes in functional circuitry in visual area MT
Abstract
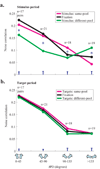
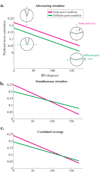
Animals can flexibly change their behavior in response to a particular sensory stimulus; the mapping between sensory and motor representations in the brain must therefore be flexible as well. Changes in the correlated firing of pairs of neurons may provide a metric of changes in functional circuitry during behavior. We studied dynamic changes in functional circuitry by analyzing the noise correlations of simultaneously recorded MT neurons in two behavioral contexts: one that promotes cooperative interactions between the two neurons and another that promotes competitive interactions. We found that identical visual stimuli give rise to differences in noise correlation in the two contexts, suggesting that MT neurons receive inputs of central origin whose strength changes with the task structure. The data are consistent with a mixed feature-based attentional strategy model in which the animal sometimes alternates attention between opposite directions of motion and sometimes attends to the two directions simultaneously.
Figures



References
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of "effective connectivity". Journal of Neurophysiology. 1989;61:900–917. - PubMed
-
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992a;257(5075):1412–1415. - PubMed
-
- Ahissar M, Ahissar E, Bergman H, Vaadia E. Encoding of sound-source location and movement: activity of single neurons and interactions between adjacent neurons in the monkey auditory cortex. J Neurophysiology. 1992b;67(1):203–215. - PubMed
-
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21(6):1399–1407. - PubMed
-
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiology. 2000;84(1):451–459. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

