Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states
- PMID: 18940731
- PMCID: PMC3847852
- DOI: 10.1016/j.stem.2008.07.027
Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states
Abstract
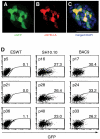
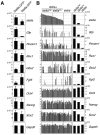
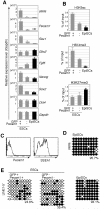
Embryonic stem cells (ESCs) are apparently homogeneous self-renewing cells, but we observed heterogeneous expression of Stella in ESCs, which is a marker of pluripotency and germ cells. Here we show that, whereas Stella-positive ESCs were like the inner cell mass (ICM), Stella-negative cells were like the epiblast cells. These states were interchangeable, which reflects the metastability and plasticity of ESCs. The established equilibrium was skewed reversibly in the absence of signals from feeder cells, which caused a marked shift toward an epiblast-like state, while trichostatin A, an inhibitor of histone deactelylase, restored Stella-positive population. The two populations also showed different histone modifications and striking functional differences, as judged by their potential for differentiation. The Stella-negative ESCs were more like the postimplantation epiblast-derived stem cells (EpiSCs), albeit the stella locus was repressed by DNA methylation in the latter, which signifies a robust epigenetic boundary between ESCs and EpiSCs.
Figures




References
-
- Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. - PubMed
-
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
-
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
