Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins
- PMID: 18940913
- PMCID: PMC3903460
- DOI: 10.1242/jcs.029769
Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins
Abstract
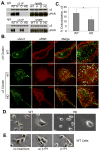
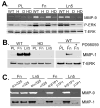
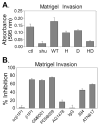
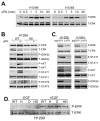
The urokinase receptor (uPAR) is upregulated upon tumor cell invasion and correlates with poor lung cancer survival. Although a cis-interaction with integrins has been ascribed to uPAR, whether this interaction alone is critical to urokinase (uPA)- and uPAR-dependent signaling and tumor promotion is unclear. Here we report the functional consequences of point mutations of uPAR (H249A-D262A) that eliminate beta1 integrin interactions but maintain uPA binding, vitronectin attachment and association with alphaV integrins, caveolin and epidermal growth factor receptor. Disruption of uPAR interactions with beta1 integrins recapitulated previously reported findings with beta1-integrin-derived peptides that attenuated matrix-dependent ERK activation, MMP expression and in vitro migration by human lung adenocarcinoma cell lines. The uPAR mutant cells acquired enhanced capacity to adhere to vitronectin via uPAR-alphaVbeta5-integrin, rather than through the uPAR-alpha3beta1-integrin complex and they were unable to initiate uPA signaling to activate ERK, Akt or Stat1. In an orthotopic lung cancer model, uPAR mutant cells exhibited reduced tumor size compared with cells expressing wild-type uPAR. Taken together, the results indicate that uPAR-beta1-integrin interactions are essential to signals induced by integrin matrix ligands or uPA that support lung cancer cell invasion in vitro and progression in vivo.
Figures



Similar articles
-
Interleukin-1alpha enhances the aggressive behavior of pancreatic cancer cells by regulating the alpha6beta1-integrin and urokinase plasminogen activator receptor expression.BMC Cell Biol. 2006 Feb 20;7:8. doi: 10.1186/1471-2121-7-8. BMC Cell Biol. 2006. PMID: 16504015 Free PMC article.
-
Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components.Cancer Res. 1997 May 1;57(9):1682-9. Cancer Res. 1997. PMID: 9135008
-
A new class of orthosteric uPAR·uPA small-molecule antagonists are allosteric inhibitors of the uPAR·vitronectin interaction.ACS Chem Biol. 2015 Jun 19;10(6):1521-34. doi: 10.1021/cb500832q. Epub 2015 Mar 31. ACS Chem Biol. 2015. PMID: 25671694 Free PMC article.
-
Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR.Stem Cells. 1997;15(6):398-408. doi: 10.1002/stem.150398. Stem Cells. 1997. PMID: 9402652 Review.
-
The urokinase-type plasminogen activator and the generation of inhibitors of urokinase activity and signaling.Curr Pharm Des. 2011;17(19):1944-61. doi: 10.2174/138161211796718143. Curr Pharm Des. 2011. PMID: 21711235 Review.
Cited by
-
Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma.PLoS One. 2010 Sep 24;5(9):e13006. doi: 10.1371/journal.pone.0013006. PLoS One. 2010. Retraction in: PLoS One. 2025 Jul 15;20(7):e0328091. doi: 10.1371/journal.pone.0328091. PMID: 20886051 Free PMC article. Retracted.
-
Design, synthesis, biochemical studies, cellular characterization, and structure-based computational studies of small molecules targeting the urokinase receptor.Bioorg Med Chem. 2012 Aug 1;20(15):4760-73. doi: 10.1016/j.bmc.2012.06.002. Epub 2012 Jun 12. Bioorg Med Chem. 2012. PMID: 22771232 Free PMC article.
-
uPAR antibody (huATN-658) and Zometa reduce breast cancer growth and skeletal lesions.Bone Res. 2020 Apr 17;8:18. doi: 10.1038/s41413-020-0094-3. eCollection 2020. Bone Res. 2020. PMID: 32337090 Free PMC article.
-
HIF-Dependent NFATC1 Activation Upregulates ITGA5 and PLAUR in Intestinal Epithelium in Inflammatory Bowel Disease.Front Genet. 2021 Nov 11;12:791640. doi: 10.3389/fgene.2021.791640. eCollection 2021. Front Genet. 2021. PMID: 34858489 Free PMC article.
-
An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo.Neoplasia. 2010 Oct;12(10):778-88. doi: 10.1593/neo.10296. Neoplasia. 2010. PMID: 20927316 Free PMC article.
References
-
- Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–159. - PubMed
-
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. - PubMed
-
- Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A, Botti G, D’Aiuto G, Salvatore M, Stoppelli MP. Urokinase receptor interacts with alpha(v)beta5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res. 1999;59:5307–5314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

