Delayed plasticity of inhibitory neurons in developing visual cortex
- PMID: 18940923
- PMCID: PMC2575499
- DOI: 10.1073/pnas.0806159105
Delayed plasticity of inhibitory neurons in developing visual cortex
Abstract
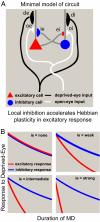
During postnatal development, altered sensory experience triggers the rapid reorganization of neuronal responses and connections in sensory neocortex. This experience-dependent plasticity is disrupted by reductions of intracortical inhibition. Little is known about how the responses of inhibitory cells themselves change during plasticity. We investigated the time course of inhibitory cell plasticity in mouse primary visual cortex by using functional two-photon microscopy with single-cell resolution and genetic identification of cell type. Initially, local inhibitory and excitatory cells had similar binocular visual response properties, both favoring the contralateral eye. After 2 days of monocular visual deprivation, excitatory cell responses shifted to favor the open eye, whereas inhibitory cells continued to respond more strongly to the deprived eye. By 4 days of deprivation, inhibitory cell responses shifted to match the faster changes in their excitatory counterparts. These findings reveal a dramatic delay in inhibitory cell plasticity. A minimal linear model reveals that the delay in inhibitory cell plasticity potently accelerates Hebbian plasticity in neighboring excitatory neurons. These findings offer a network-level explanation as to how inhibition regulates the experience-dependent plasticity of neocortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. - PubMed
-
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. - PubMed
-
- Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

