Acid phosphatase 5 is responsible for removing the mannose 6-phosphate recognition marker from lysosomal proteins
- PMID: 18940929
- PMCID: PMC2575464
- DOI: 10.1073/pnas.0807472105
Acid phosphatase 5 is responsible for removing the mannose 6-phosphate recognition marker from lysosomal proteins
Abstract
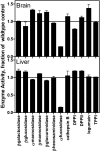
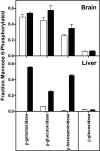
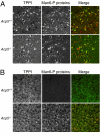
Most newly synthesized proteins destined for the lysosome reach this location via a specific intracellular pathway. In the Golgi, a phosphotransferase specifically labels lysosomal proteins with mannose 6-phosphate (Man-6-P). This modification is recognized by receptors that target the lysosomal proteins to the lysosome where, in most cell types, the Man-6-P recognition marker is rapidly removed. Despite extensive characterization of this pathway, the enzyme responsible for the removal of the targeting modification has remained elusive. In this study, we have identified this activity. Preliminary investigations using a cell-based bioassay were used to follow a dephosphorylation activity that was associated with the lysosomal fraction. This activity was high in the liver, where endogenous lysosomal proteins are efficiently dephosphorylated, but present at a much lower level in the brain, where the modification persists. This observation, combined with an analysis of the expression of lysosomal proteins in different tissues, led us to identify acid phosphatase 5 (ACP5) as a candidate for the enzyme that removes Man-6-P. Expression of ACP5 in N1E-115 neuroblastoma cells, which do not efficiently dephosphorylate lysosomal proteins, significantly decreased the steady state levels of Man6-P glycoproteins. Analysis of ACP5-deficient mice revealed that levels of Man-6-P glycoproteins were highly elevated in tissues that normally express ACP5, and this resulted from a failure to dephosphorylate lysosomal proteins. These results indicate a central role for ACP5 in removal of the Man-6-P recognition marker and open up new avenues to investigate the importance of this process in cell biology and medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mannose 6 dephosphorylation of lysosomal proteins mediated by acid phosphatases Acp2 and Acp5.Mol Cell Biol. 2012 Feb;32(4):774-82. doi: 10.1128/MCB.06195-11. Epub 2011 Dec 12. Mol Cell Biol. 2012. PMID: 22158965 Free PMC article.
-
Overlapping functions of lysosomal acid phosphatase (LAP) and tartrate-resistant acid phosphatase (Acp5) revealed by doubly deficient mice.Development. 2001 Dec;128(23):4899-910. doi: 10.1242/dev.128.23.4899. Development. 2001. PMID: 11731469
-
Extending the mannose 6-phosphate glycoproteome by high resolution/accuracy mass spectrometry analysis of control and acid phosphatase 5-deficient mice.Mol Cell Proteomics. 2013 Jul;12(7):1806-17. doi: 10.1074/mcp.M112.026179. Epub 2013 Mar 11. Mol Cell Proteomics. 2013. PMID: 23478313 Free PMC article.
-
A shortcut to the lysosome: the mannose-6-phosphate-independent pathway.Mol Genet Metab. 2012 Nov;107(3):257-66. doi: 10.1016/j.ymgme.2012.07.012. Epub 2012 Jul 20. Mol Genet Metab. 2012. PMID: 22884962 Review.
-
Tartrate-resistant acid phosphatase knockout mice.J Bone Miner Res. 2003 Oct;18(10):1905-7. doi: 10.1359/jbmr.2003.18.10.1905. J Bone Miner Res. 2003. PMID: 14584904 Review.
Cited by
-
Analysis of Brain and Cerebrospinal Fluid from Mouse Models of the Three Major Forms of Neuronal Ceroid Lipofuscinosis Reveals Changes in the Lysosomal Proteome.Mol Cell Proteomics. 2019 Nov;18(11):2244-2261. doi: 10.1074/mcp.RA119.001587. Epub 2019 Sep 9. Mol Cell Proteomics. 2019. PMID: 31501224 Free PMC article.
-
Monocytes/Macrophages Upregulate the Hyaluronidase HYAL1 and Adapt Its Subcellular Trafficking to Promote Extracellular Residency upon Differentiation into Osteoclasts.PLoS One. 2016 Oct 18;11(10):e0165004. doi: 10.1371/journal.pone.0165004. eCollection 2016. PLoS One. 2016. PMID: 27755597 Free PMC article.
-
Mannose 6 dephosphorylation of lysosomal proteins mediated by acid phosphatases Acp2 and Acp5.Mol Cell Biol. 2012 Feb;32(4):774-82. doi: 10.1128/MCB.06195-11. Epub 2011 Dec 12. Mol Cell Biol. 2012. PMID: 22158965 Free PMC article.
-
Vitamin D3 and carbamazepine protect against Clostridioides difficile infection in mice by restoring macrophage lysosome acidification.Autophagy. 2022 Sep;18(9):2050-2067. doi: 10.1080/15548627.2021.2016004. Epub 2022 Jan 6. Autophagy. 2022. PMID: 34989311 Free PMC article.
-
Mass spectrometry-based protein profiling to determine the cause of lysosomal storage diseases of unknown etiology.Mol Cell Proteomics. 2009 Jul;8(7):1708-18. doi: 10.1074/mcp.M900122-MCP200. Epub 2009 Apr 20. Mol Cell Proteomics. 2009. PMID: 19383612 Free PMC article.
References
-
- Holtzman E. Lysosomes. New York: Plenum Press; 1989.
-
- Kornfeld S, Sly WS. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, et al., editors. New York: McGraw–Hill; 2001. pp. 3469–3482.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

