Tetrodotoxin: a brief history
- PMID: 18941294
- PMCID: PMC2858367
- DOI: 10.2183/pjab.84.147
Tetrodotoxin: a brief history
Abstract
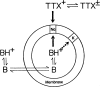
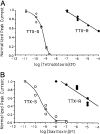
Tetrodotoxin (TTX), contained in puffer, has become an extremely popular chemical tool in the physiological and pharmacological laboratories since our discovery of its channel blocking action in the early 1960s. This brief review describes the history of discovery of TTX action on sodium channels, and represents a story primarily of my own work. TTX inhibits voltage-gated sodium channels in a highly potent and selective manner without effects on any other receptor and ion channel systems. TTX blocks the sodium channel only from outside of the nerve membrane, and is due to binding to the selectivity filter resulting in prevention of sodium ion flow. It does not impairs the channel gating mechanism. More recently, the TTX-resistant sodium channels have been discovered in the nervous system and received much attention because of their role in pain sensation. TTX is now known to be produced not by puffer but by bacteria, and reaches various species of animals via food chain.(Communicated by Masanori OTSUKA, M.J.A.).
Figures

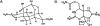


References
-
- Noguchi, T. and Ebesu, J.S.M. (2001) J. Toxicol.-Toxin Rev. 20, 1–10
-
- Narahashi, T., Deguchi, T., Urakawa, N. and Ohkubo, Y. (1960) Amer. J. Physiol. 198, 934–938 - PubMed
-
- Nastuk, W.L. and Hodgkin, A.L. (1950) J. Cell. Comp. Physiol. 35, 39–74
-
- Urakawa, N., Narahashi, T., Deguchi, T. and Ohkubo, Y. (1960) Amer. J. Physiol. 198, 938–942 - PubMed

