Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia
- PMID: 18941301
- PMCID: PMC3666648
- DOI: 10.2183/pjab.84.246
Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia
Abstract
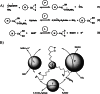
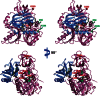
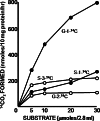
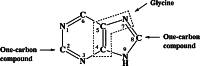
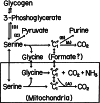
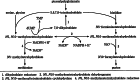
The glycine cleavage system catalyzes the following reversible reaction: Glycine + H(4)folate + NAD(+) <==> 5,10-methylene-H(4)folate + CO(2) + NH(3) + NADH + H(+)The glycine cleavage system is widely distributed in animals, plants and bacteria and consists of three intrinsic and one common components: those are i) P-protein, a pyridoxal phosphate-containing protein, ii) T-protein, a protein required for the tetrahydrofolate-dependent reaction, iii) H-protein, a protein that carries the aminomethyl intermediate and then hydrogen through the prosthetic lipoyl moiety, and iv) L-protein, a common lipoamide dehydrogenase. In animals and plants, the proteins form an enzyme complex loosely associating with the mitochondrial inner membrane. In the enzymatic reaction, H-protein converts P-protein, which is by itself a potential alpha-amino acid decarboxylase, to an active enzyme, and also forms a complex with T-protein. In both glycine cleavage and synthesis, aminomethyl moiety bound to lipoic acid of H-protein represents the intermediate that is degraded to or can be formed from N(5),N(10)-methylene-H(4)folate and ammonia by the action of T-protein. N(5),N(10)-Methylene-H(4)folate is used for the biosynthesis of various cellular substances such as purines, thymidylate and methionine that is the major methyl group donor through S-adenosyl-methionine. This accounts for the physiological importance of the glycine cleavage system as the most prominent pathway in serine and glycine catabolism in various vertebrates including humans. Nonketotic hyperglycinemia, a congenital metabolic disorder in human infants, results from defective glycine cleavage activity. The majority of patients with nonketotic hyperglycinemia had lesions in the P-protein gene, whereas some had mutant T-protein genes. The only patient classified into the degenerative type of nonketotic hyperglycinemia had an H-protein devoid of the prosthetic lipoyl residue. The crystallography of normal T-protein as well as biochemical characterization of recombinants of the normal and mutant T-proteins confirmed why the mutant T-proteins had lost enzyme activity. Putative mechanisms of cellular injuries including those in the central nervous system of patients with nonketotic hyperglycinemia are discussed.
Figures


References
-
- Richert, D. A., Amberg, R. and Wilson, M. (1962) Metabolism of glycine by avian liver. J. Biol. Chem. 237, 99–103 - PubMed
-
- Kawasaki, H., Sato, T. and Kikuchi, G. (1966) A new reaction for glycine biosynthesis. Biochem. Biophys. Res. Commun. 23, 227–233 - PubMed
-
- Sato, T., Kochi, H., Motokawa, Y., Kawasaki, H. and Kikuchi, G. (1969) Glycine metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J. Biochem. 65, 63–70 - PubMed
-
- Motokawa, Y. and Kikuchi, G. (1969) Glycine metabolism by rat liver mitochondria. II. Methylene tetrahydrofolate as the direct one carbon donor in the reaction of glycine synthesis. J. Biochem. 65, 71–75 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

