Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of alpha-1-antitrypsin using invasive and noninvasive delivery
- PMID: 18941444
- PMCID: PMC2834977
- DOI: 10.1038/mt.2008.217
Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of alpha-1-antitrypsin using invasive and noninvasive delivery
Abstract
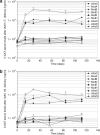
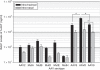
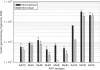
Recombinant adeno-associated viral (rAAV) vectors have been widely used in pulmonary gene therapy research. In this study, we evaluated the transduction and expression efficiencies of several AAV serotypes and AAV2 capsid mutants with specific pulmonary targeting ligands in the mouse lung. The noninvasive intranasal delivery was compared with the traditional intratracheal lung delivery. The rAAV8 was the most efficient serotype at expressing alpha-1-antitrypsin (AAT) in the lung among all the tested serotypes and mutants. A dose of 1 x 10(10) vg of rAAV8-CB-AAT transduced a high percentage of cells in the lung when delivered intratrachealy. The serum and the broncho-alveolar lavage fluid (BALF) levels of human AAT (hAAT) were about 6- and 2.5-fold higher, respectively, than those of rAAV5 group. Among the rAAV2 capsid mutants, the rAAV2 capsid mutants that display a peptide sequence from hAAT ("long serpin") indicated a twofold increase in transgene expression. For most vectors, the serum hAAT levels achieved after intranasal delivery were 1/2 to 1/3 of those with the intratracheal method. Overall, rAAV8 was the most promising vector for the future application in gene therapy of pulmonary diseases such as AAT deficiency-related emphysema.
Figures


Similar articles
-
Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector.J Gene Med. 2006 Jun;8(6):730-5. doi: 10.1002/jgm.896. J Gene Med. 2006. PMID: 16518879
-
Enhancing rAAV vector expression in the lung.J Gene Med. 2005 Jul;7(7):842-50. doi: 10.1002/jgm.759. J Gene Med. 2005. PMID: 15838934
-
Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs.Mol Ther. 2010 Jun;18(6):1165-72. doi: 10.1038/mt.2010.51. Epub 2010 Apr 6. Mol Ther. 2010. PMID: 20372105 Free PMC article.
-
Gene therapy progress and prospects: alpha-1 antitrypsin.Gene Ther. 2003 Jan;10(2):95-9. doi: 10.1038/sj.gt.3301947. Gene Ther. 2003. PMID: 12571637 Review.
-
Current status of gene therapy for α-1 antitrypsin deficiency.Expert Opin Biol Ther. 2015 Mar;15(3):329-36. doi: 10.1517/14712598.2015.978854. Epub 2014 Nov 3. Expert Opin Biol Ther. 2015. PMID: 25363251 Review.
Cited by
-
Advanced approaches to overcome biological barriers in respiratory and systemic routes of administration for enhanced nucleic acid delivery to the lung.Expert Opin Drug Deliv. 2023 Jul-Dec;20(11):1531-1552. doi: 10.1080/17425247.2023.2282535. Epub 2023 Dec 20. Expert Opin Drug Deliv. 2023. PMID: 37946533 Free PMC article. Review.
-
Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here?Annu Rev Virol. 2019 Sep 29;6(1):601-621. doi: 10.1146/annurev-virology-092818-015530. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283441 Free PMC article.
-
AAV's anatomy: roadmap for optimizing vectors for translational success.Curr Gene Ther. 2010 Oct;10(5):319-340. doi: 10.2174/156652310793180706. Curr Gene Ther. 2010. PMID: 20712583 Free PMC article.
-
Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method.Hum Gene Ther. 2011 Feb;22(2):155-65. doi: 10.1089/hum.2010.118. Epub 2010 Dec 12. Hum Gene Ther. 2011. PMID: 20812844 Free PMC article.
-
Effect of cigarette smoke exposure and structural modifications on the α-1 Antitrypsin interaction with caspases.Mol Med. 2012 May 9;18(1):445-54. doi: 10.2119/molmed.2011.00207. Mol Med. 2012. PMID: 22245800 Free PMC article.
References
-
- Flotte TR. Recent developments in recombinant AAV-mediated gene therapy for lung diseases. Curr Gene Ther. 2005;5:361–366. - PubMed
-
- Flotte TR. Adeno-associated virus-based gene therapy for inherited disorders. Pediatr Res. 2005;58:1143–1147. - PubMed
-
- Flotte TR. Gene therapy progress and prospects: recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2004;11:805–810. - PubMed
-
- Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A, et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10:1551–1558. - PubMed
-
- Virella-Lowell I, Zusman B, Foust K, Loiler S, Conlon T, Song S, et al. Enhancing rAAV vector expression in the lung. J Gene Med. 2005;7:842–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

