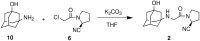Synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile: a key intermediate for dipeptidyl peptidase IV inhibitors
- PMID: 18941490
- PMCID: PMC2486489
- DOI: 10.3762/bjoc.4.20
Synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile: a key intermediate for dipeptidyl peptidase IV inhibitors
Abstract
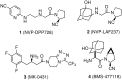
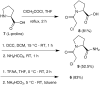
An alternative and practical synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile was achieved. Reaction of L-proline with chloroacetyl chloride was followed by conversion of the carboxylic acid moiety of the resulting N-acylated product into the carbonitrile via the corresponding amide intermediate. The synthesized pyrrolidine derivative was utilized to prepare DPP-IV inhibitor Vildagliptin.
Keywords: 2(S)-cyanopyrrolidine; DPP-IV inhibitors; L-proline; N-acylation; amides.
Figures
Similar articles
-
Synthesis and biological evaluation of pyrrolidine-2-carbonitrile and 4-fluoropyrrolidine-2-carbonitrile derivatives as dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes.Bioorg Med Chem. 2013 Dec 1;21(23):7418-29. doi: 10.1016/j.bmc.2013.09.048. Epub 2013 Oct 1. Bioorg Med Chem. 2013. PMID: 24153396
-
Synthesis and evaluation of structurally constrained imidazolidin derivatives as potent dipeptidyl peptidase IV inhibitors.Eur J Med Chem. 2009 Aug;44(8):3318-22. doi: 10.1016/j.ejmech.2009.03.021. Epub 2009 Mar 26. Eur J Med Chem. 2009. PMID: 19375196
-
Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by Vildagliptin ((2S)-{[(3-hydroxyadamantan-1-yl)amino]acetyl}-pyrrolidine-2-carbonitrile).Biochem Pharmacol. 2005 Jul 1;70(1):134-43. doi: 10.1016/j.bcp.2005.04.009. Biochem Pharmacol. 2005. PMID: 15907807
-
The therapeutic potential of inhibitors of dipeptidyl peptidase IV (DPP IV) and related proline-specific dipeptidyl aminopeptidases.Curr Med Chem. 2005;12(8):971-98. doi: 10.2174/0929867053507298. Curr Med Chem. 2005. PMID: 15853709 Review.
-
11 Years of cyanopyrrolidines as DPP-IV inhibitors.Curr Top Med Chem. 2007;7(6):579-95. doi: 10.2174/156802607780091000. Curr Top Med Chem. 2007. PMID: 17352679 Review.
Cited by
-
Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4).Pharmaceuticals (Basel). 2022 Feb 22;15(3):273. doi: 10.3390/ph15030273. Pharmaceuticals (Basel). 2022. PMID: 35337071 Free PMC article.
-
Progress in the Stereoselective Synthesis Methods of Pyrrolidine-Containing Drugs and Their Precursors.Int J Mol Sci. 2024 Oct 17;25(20):11158. doi: 10.3390/ijms252011158. Int J Mol Sci. 2024. PMID: 39456938 Free PMC article. Review.
-
Synthesis of imidazo-1,4-oxazinone derivatives and investigation of reaction mechanism.Turk J Chem. 2021 Oct 19;45(5):1639-1649. doi: 10.3906/kim-2106-28. eCollection 2021. Turk J Chem. 2021. PMID: 34849073 Free PMC article.
-
Liquid chromatographic methods for the determination of vildagliptin in the presence of its synthetic intermediate and the simultaneous determination of pioglitazone hydrochloride and metformin hydrochloride.Int J Biomed Sci. 2011 Sep;7(3):201-8. Int J Biomed Sci. 2011. PMID: 23675237 Free PMC article.
-
Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents.Molecules. 2023 Aug 3;28(15):5860. doi: 10.3390/molecules28155860. Molecules. 2023. PMID: 37570832 Free PMC article. Review.
References
-
- Mest H-J. Curr Opin Invest Drugs. 2006;7:338–343. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources