Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements
- PMID: 18941507
- PMCID: PMC2565064
- DOI: 10.1371/journal.pone.0003439
Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements
Abstract
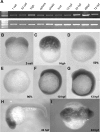
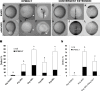
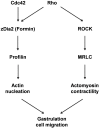
Intensive cellular movements occur during gastrulation. These cellular movements rely heavily on dynamic actin assembly. Rho with its associated proteins, including the Rho-activated formin, Diaphanous, are key regulators of actin assembly in cellular protrusion and migration. However, the function of Diaphanous in gastrulation cell movements remains unclear. To study the role of Diaphanous in gastrulation, we isolated a partial zebrafish diaphanous-related formin 2 (zdia2) clone with its N-terminal regulatory domains. The GTPase binding domain of zDia2 is highly conserved compared to its mammalian homologues. Using a yeast two-hybrid assay, we showed that zDia2 interacts with constitutively-active RhoA and Cdc42. The zdia2 mRNAs were ubiquitously expressed during early embryonic development in zebrafish as determined by RT-PCR and whole-mount in situ hybridization analyses. Knockdown of zdia2 by antisense morpholino oligonucleotides (MOs) blocked epiboly formation and convergent extension in a dose-dependent manner, whereas ectopic expression of a human mdia gene partially rescued these defects. Time-lapse recording further showed that bleb-like cellular processes of blastoderm marginal deep marginal cells and pseudopod-/filopod-like processes of prechordal plate cells and lateral cells were abolished in the zdia2 morphants. Furthermore, zDia2 acts cell-autonomously since transplanted zdia2-knockdown cells exhibited low protrusive activity with aberrant migration in wild type host embryos. Lastly, co-injection of antisense MOs of zdia2 and zebrafish profilin I (zpfn 1), but not zebrafish profilin II, resulted in a synergistic inhibition of gastrulation cell movements. These results suggest that zDia2 in conjunction with zPfn 1 are required for gastrulation cell movements in zebrafish.
Conflict of interest statement
Figures


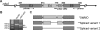





Similar articles
-
RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling.Cell Signal. 2006 Mar;18(3):359-72. doi: 10.1016/j.cellsig.2005.05.019. Epub 2005 Jul 14. Cell Signal. 2006. PMID: 16019189
-
Ptenb mediates gastrulation cell movements via Cdc42/AKT1 in zebrafish.PLoS One. 2011 Apr 11;6(4):e18702. doi: 10.1371/journal.pone.0018702. PLoS One. 2011. PMID: 21494560 Free PMC article.
-
The zebrafish band 4.1 member Mir is involved in cell movements associated with gastrulation.Dev Biol. 2003 Dec 15;264(2):407-29. doi: 10.1016/j.ydbio.2003.09.001. Dev Biol. 2003. PMID: 14651927
-
[Zebra fish cell movements during gastrulation].Yi Chuan. 2013 Apr;35(4):441-8. doi: 10.3724/sp.j.1005.2013.00441. Yi Chuan. 2013. PMID: 23659934 Review. Chinese.
-
Zebrafish gastrulation: cell movements, signals, and mechanisms.Int Rev Cytol. 2007;261:159-92. doi: 10.1016/S0074-7696(07)61004-3. Int Rev Cytol. 2007. PMID: 17560282 Review.
Cited by
-
Profilin choreographs actin and microtubules in cells and cancer.Int Rev Cell Mol Biol. 2020;355:155-204. doi: 10.1016/bs.ircmb.2020.05.005. Epub 2020 Jul 16. Int Rev Cell Mol Biol. 2020. PMID: 32859370 Free PMC article.
-
Formins as effector proteins of Rho GTPases.Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914801 Free PMC article. Review.
-
Dia-interacting protein (DIP) imposes migratory plasticity in mDia2-dependent tumor cells in three-dimensional matrices.PLoS One. 2012;7(9):e45085. doi: 10.1371/journal.pone.0045085. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024796 Free PMC article.
-
Cellular and molecular mechanisms of convergence and extension in zebrafish.Curr Top Dev Biol. 2020;136:377-407. doi: 10.1016/bs.ctdb.2019.08.001. Epub 2019 Sep 3. Curr Top Dev Biol. 2020. PMID: 31959296 Free PMC article. Review.
-
Models of convergent extension during morphogenesis.Wiley Interdiscip Rev Dev Biol. 2018 Jan;7(1):e293. doi: 10.1002/wdev.293. Epub 2017 Sep 14. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 28906063 Free PMC article. Review.
References
-
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–R228. - PubMed
-
- Schier AF. Axis formation and patterning in zebrafish. Curr Opin Genet Dev. 2001;11:393–404. - PubMed
-
- Solnica-Krezel L. Gastrulation in zebrafish – all just about adhesion? Curr Opin Genet Dev. 2006;16:433–441. - PubMed
-
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

