Signaling pathways regulating the expression of Prx1 and Prx2 in the chick mandibular mesenchyme
- PMID: 18942149
- PMCID: PMC2718419
- DOI: 10.1002/dvdy.21762
Signaling pathways regulating the expression of Prx1 and Prx2 in the chick mandibular mesenchyme
Abstract
Prx1 and Prx2 are members of the aristaless-related homeobox genes shown to play redundant but essential roles in morphogenesis of the mandibular processes. To gain insight into the signaling pathways that regulate expression of Prx genes in the mandibular mesenchyme, we used the chick as a model system. We examined the patterns of gene expression in the face and the roles of signals derived from the epithelium on the expression of Prx genes in the mandibular mesenchyme. Our results demonstrated stage-dependent roles of mandibular epithelium on the expression of Prx in the mandibular mesenchyme and provide evidence for positive roles of members of the fibroblast and hedgehog families derived from mandibular epithelium on the expression of Prx genes in the mandibular mesenchyme. Our studies suggest that endothelin-1 signaling derived from the mesenchyme is involved in restricting the expression of Prx2 to the medial mandibular mesenchyme.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures

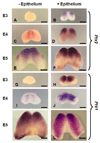


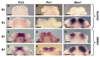
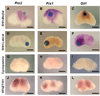
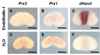

References
-
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. - PubMed
-
- Barlow AJ, Bogardi JP, Ladher R, Francis-West PH. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev Dyn. 1999;214:291–302. - PubMed
-
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. - PubMed
-
- Buxton P, Davey MG, Paton IR, Morrice DR, Francis-West PH, Burt DW, Tickle C. Craniofacial development in the talpid3 chicken mutant. Differentiation. 2004;72:348–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

