Promotion of reprogramming to ground state pluripotency by signal inhibition
- PMID: 18942890
- PMCID: PMC2570424
- DOI: 10.1371/journal.pbio.0060253
Promotion of reprogramming to ground state pluripotency by signal inhibition
Abstract
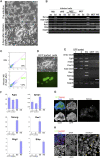
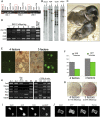
Induced pluripotent stem (iPS) cells are generated from somatic cells by genetic manipulation. Reprogramming entails multiple transgene integrations and occurs apparently stochastically in rare cells over many days. Tissue stem cells may be subject to less-stringent epigenetic restrictions than other cells and might therefore be more amenable to deprogramming. We report that brain-derived neural stem (NS) cells acquire undifferentiated morphology rapidly and at high frequency after a single round of transduction with reprogramming factors. However, critical attributes of true pluripotency--including stable expression of endogenous Oct4 and Nanog, epigenetic erasure of X chromosome silencing in female cells, and ability to colonise chimaeras--were not attained. We therefore applied molecularly defined conditions for the derivation and propagation of authentic pluripotent stem cells from embryos. We combined dual inhibition (2i) of mitogen-activated protein kinase signalling and glycogen synthase kinase-3 (GSK3) with the self-renewal cytokine leukaemia inhibitory factor (LIF). The 2i/LIF condition induced stable up-regulation of Oct4 and Nanog, reactivation of the X chromosome, transgene silencing, and competence for somatic and germline chimaerism. Using 2i /LIF, NS cell reprogramming required only 1-2 integrations of each transgene. Furthermore, transduction with Sox2 and c-Myc is dispensable, and Oct4 and Klf4 are sufficient to convert NS cells into chimaera-forming iPS cells. These findings demonstrate that somatic cell state influences requirements for reprogramming and delineate two phases in the process. The ability to capture pre-pluripotent cells that can advance to ground state pluripotency simply and with high efficiency opens a door to molecular dissection of this remarkable phenomenon.
Conflict of interest statement
Figures




Comment in
-
A shortcut to immortality: rapid reprogramming with tissue cells.PLoS Biol. 2008 Oct;6(10):e275. doi: 10.1371/journal.pbio.0060275. Epub 2008 Oct 21. PLoS Biol. 2008. PMID: 20076693 Free PMC article. No abstract available.
References
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

