Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components
- PMID: 18945674
- PMCID: PMC2602914
- DOI: 10.1074/jbc.M804543200
Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components
Abstract
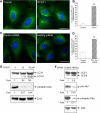
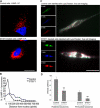
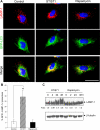
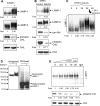
Autophagy is a lysosome-dependent degradative pathway that regulates the turnover of intracellular organelles, parasites, and long-lived proteins. Deregulation of autophagy results in a variety of pathological conditions, but little is known regarding the mechanisms that link normal cellular and pathological signals to the regulation of distinct stages in the autophagy pathway. Here we uncover a novel role for the Abl family kinases in the regulation of the late stages of autophagy. Inhibition, depletion, or knockout of the Abl family kinases, Abl and Arg, resulted in a dramatic reduction in the intracellular activities of the lysosomal glycosidases alpha-galactosidase, alpha-mannosidase and neuraminidase. Inhibition of Abl kinases also reduced the processing of the precursor forms of cathepsin D and cathepsin L to their mature, lysosomal forms, which coincided with the impaired turnover of long-lived cytosolic proteins and accumulation of autophagosomes. Furthermore, defective lysosomal degradation of long-lived proteins in the absence of Abl kinase signaling was accompanied by a perinuclear redistribution of lysosomes and increased glycosylation and stability of lysosome-associated membrane proteins, which are known to be substrates for lysosomal enzymes and play a role in regulating lysosome mobility. Our findings reveal a role for Abl kinases in the regulation of late-stage autophagy and have important implications for therapies that employ pharmacological inhibitors of the Abl kinases.
Figures


References
-
- Rubinsztein, D. C., Gestwicki, J. E., Murphy, L. O., and Klionsky, D. J. (2007) Nat. Rev. Drug Discov. 6 304-312 - PubMed
-
- Meijer, A. J., and Codogno, P. (2004) Int. J. Biochem. Cell Biol. 36 2445-2462 - PubMed
-
- Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005) Science 307 1098-1101 - PubMed
-
- Maiuri, M. C., Zalckvar, E., Kimchi, A., and Kroemer, G. (2007) Nat. Rev. Mol. Cell. Biol. 8 741-752 - PubMed
-
- Guertin, D. A., and Sabatini, D. M. (2007) Cancer Cell 12 9-22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

