Suppressors of the cdc-25.1(gf)-associated intestinal hyperplasia reveal important maternal roles for prp-8 and a subset of splicing factors in C. elegans
- PMID: 18945809
- PMCID: PMC2590948
- DOI: 10.1261/rna.1168408
Suppressors of the cdc-25.1(gf)-associated intestinal hyperplasia reveal important maternal roles for prp-8 and a subset of splicing factors in C. elegans
Abstract
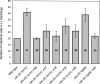
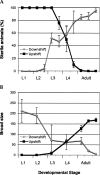
The maternal contribution of gene products enables embryos to initiate their developmental program in the absence of zygotic gene expression. In Caenorhabditis elegans, maternal CDC-25.1 levels are tightly regulated to promote early cell divisions, while stabilization of this phosphatase by gain-of-function mutations gives rise to intestinal-specific hyperplasia. To identify regulators of CDC-25.1 levels and/or function, we performed a modifier screen of the cdc-25.1(gf)-dependent hyperplasia. One of the isolated suppressor mutants possesses a donor splice site mutation in prp-8, a key splicing factor of the U5-specific snRNP. prp-8(rr40) produces aberrant prp-8 splice variants that generate C-terminal truncations at the expense of wild-type prp-8. Levels of maternal transcripts are reduced, including cdc-25.1, while zygotic transcripts appear unperturbed, suggesting a germ-line-specific role for this splicing factor in regulating the splicing, and consequently, the steady-state levels of maternal transcripts. Using a novel feeding RNAi strategy we found that only a subset of splicing factors suppress cdc-25.1(gf), suggesting that they too may play specific roles in germ-line spliceosome function. In humans, mutations in the corresponding hPrp8 C-terminal domain result in retinitis pigmentosa, a retinal-specific disorder. Intriguingly, despite affecting the general splicing apparatus, both human and C. elegans show tissue-specific defects resulting from mutations in this key splicing component. Our findings suggest that in addition to its important regulatory function in the C. elegans germ line, prp-8(rr40) may provide further insight into the etiology of this splicing-associated human disorder.
Figures



References
-
- Alphey L., Jimenez J., White-Cooper H., Dawson I., Nurse P., Glover D.M. twine, a cdc25 homolog that functions in the male and female germ line of Drosophila . Cell. 1992;69:977–988. - PubMed
-
- Ashcroft N., Golden A. CDC-25.1 regulates germ line proliferation in Caenorhabditis elegans . Genesis. 2002;33:1–7. - PubMed
-
- Ashcroft N.R., Kosinski M.E., Wickramasinghe D., Donovan P.J., Golden A. The four cdc25 genes from the nematode Caenorhabditis elegans . Gene. 1998;214:59–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
