Topology of class A G protein-coupled receptors: insights gained from crystal structures of rhodopsins, adrenergic and adenosine receptors
- PMID: 18945819
- PMCID: PMC2652756
- DOI: 10.1124/mol.108.051938
Topology of class A G protein-coupled receptors: insights gained from crystal structures of rhodopsins, adrenergic and adenosine receptors
Abstract
Biological membranes are densely packed with membrane proteins that occupy approximately half of their volume. In almost all cases, membrane proteins in the native state lack the higher-order symmetry required for their direct study by diffraction methods. Despite many technical difficulties, numerous crystal structures of detergent solubilized membrane proteins have been determined that illustrate their internal organization. Among such proteins, class A G protein-coupled receptors have become amenable to crystallization and high resolution X-ray diffraction analyses. The derived structures of native and engineered receptors not only provide insights into their molecular arrangements but also furnish a framework for designing and testing potential models of transformation from inactive to active receptor signaling states and for initiating rational drug design.
Figures
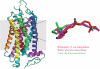
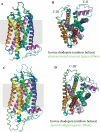
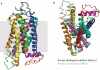



References
-
- Becker OM, Shacham S, Marantz Y, and Noiman S (2003) Modeling the 3D structure of GPCRs: advances and application to drug discovery. Curr Opin Drug Discov Devel 6 353-361. - PubMed
-
- Buczylko J, Saari JC, Crouch RK, and Palczewski K (1996) Mechanisms of opsin activation. J Biol Chem 271 20621-20630. - PubMed
-
- Chabre M, Cone R, and Saibil H (2003) Biophysics: is rhodopsin dimeric in native retinal rods? Nature 426 30-31. - PubMed
-
- Civelli O (2005) GPCR deorphanizations: the novel, the known and the unexpected transmitters. Trends Pharmacol Sci 26 15-19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

