Swiss Cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3
- PMID: 18945896
- PMCID: PMC2723165
- DOI: 10.1523/JNEUROSCI.3015-08.2008
Swiss Cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3
Abstract
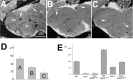
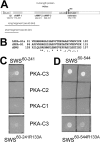
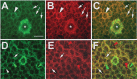
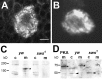
The Drosophila Swiss Cheese (SWS) protein and its vertebrate ortholog Neuropathy Target Esterase (NTE) are required for neuronal survival and glial integrity. In humans, NTE is the target of organophosphorous compounds which cause a paralyzing axonal degeneration and recently mutations in NTE have been shown to cause a Hereditary Spastic Paraplegia called NTE-related Motor-Neuron Disorder. SWS and NTE are concentrated in the endoplasmic reticulum and both have been shown to have an esterase function against an artificial substrate. However, the functional mechanisms and the pathways in which SWS/NTE are involved in are still widely unknown. Here, we show that SWS interacts specifically with the C3 catalytic subunit of cAMP activated protein kinase (PKA-C3), which together with orthologs in mouse (Pkare) and human (PrKX) forms a novel class of catalytic subunits of unknown function. This interaction requires a domain of SWS which shows homology to regulatory subunits of PKA and, like conventional regulatory subunits, the binding of SWS to the PKA-C3 inhibits its function. Consistent with this result, expression of additional PKA-C3 induces degeneration and enhances the neurodegenerative phenotype in sws mutants. We also show that the complex formation with the membrane-bound SWS tethers PKA-C3 to membranes. We therefore propose a model in which SWS acts as a noncanonical subunit for PKA-C3, whereby the complex formation regulates the localization and kinase activity of PKA-C3, and that disruption of this regulation can induce neurodegeneration.
Figures


References
-
- Bettencourt da Cruz A, Schwärzel M, Schulze S, Niyyati M, Heisenberg M, Kretzschmar D. Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila. Mol Biol Cell. 2005;16:2433–2442. - PMC - PubMed
-
- Blaschke RJ, Monaghan AP, Bock D, Rappold GA. A novel murine PKA-related protein kinase involved in neuronal differentiation. Genomics. 2000;64:187–194. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
