Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex
- PMID: 18945905
- PMCID: PMC3981993
- DOI: 10.1523/JNEUROSCI.1521-08.2008
Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex
Abstract
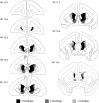
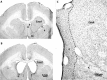
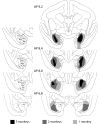
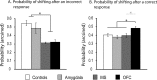
The ability to switch responding between two visual stimuli based on their changing relationship with reward is dependent on the orbitofrontal cortex (OFC). OFC lesions in humans, monkeys, and rats disrupt performance on a common test of this ability, the visual serial discrimination reversal task. This finding is of particular significance to our understanding of psychiatric disorders such as obsessive-compulsive disorder (OCD) and schizophrenia, in which behavioral inflexibility is a prominent symptom. Although OFC dysfunction can occur in these disorders, there is considerable evidence for more widespread dysfunction within frontostriatal and frontoamygdalar circuitry. Because the contribution of these subcortical structures to behavioral flexibility is poorly understood, the present study compared the effects of excitotoxic lesions of the medial striatum (MS), amygdala, and OFC in the marmoset monkey on performance of the serial reversal task. All monkeys were able to learn a novel stimulus-reward association but, compared with both control and amygdala-lesioned monkeys, those with MS or OFC lesions showed a perseverative impairment in their ability to reverse this association. However, whereas both MS and OFC groups showed insensitivity to negative feedback, only OFC-lesioned monkeys showed insensitivity to positive feedback. These findings suggest that, for different reasons, both the MS and OFC support behavioral flexibility after changes in reward contingencies, and are consistent with the hypothesis that striatal and OFC dysfunction can contribute to pathological perseveration.
Figures




References
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits-parallel substrates for motor, oculomotor, prefrontal and limbic functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Annett LE, McGregor A, Robbins TW. The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behav Brain Res. 1989;31:231–242. - PubMed
-
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. - PubMed
-
- Burns LH, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the basolateral amygdala on conditional discrimination learning with primary and conditioned reinforcement. Behav Brain Res. 1999;100:123–133. - PubMed
-
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
